Fungi - pigeonpea
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Scientific name
Colletotrichum cajani Rangel.
Importance
High.
Significance
Anthracnose is destructive and infects leaves, pods and seeds especially during the period of heavy rainfall. The losses are mainly due to dropping of pods at young stage and discoloration and decay of some or all the seeds in the infected pods. In a harvest of affected crop, 86% pods have been found to be infected with 36% unmarketable seeds (Tucker 1927).
Symptoms
The typical symptoms are sunken spots on pods due to drying up and collapse of the cells in the center. The presence of pink sticky spore masses that develop during the moist weather have quite an ulcer like appearance. The main symptoms include spots on pods and leaves, blackening and shrinking of veins of infected leaves and their premature fall. The infected pods become distorted, abort and die. Affected young pods drop and all the seeds in the infected pods are discolored and decayed. Anthracnose spots appear as irregular, brown to grayish stem lesions containing dark, scattered acervuli. Severe infection leads to drying and death of infected branches. Minute spots with dark margins and gray centers scattered with acrvuli are also common on the leaves and pods of infected plants (Reddy et al. 1993).
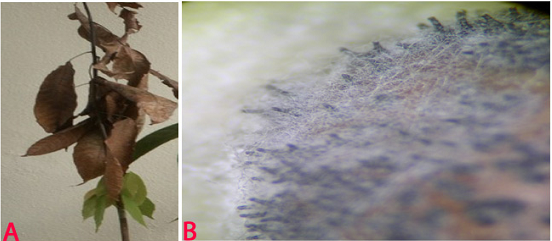
 Anthracnose (Colletotrichum cajani) of pigeonpea: (A)leasions on plant and pods (photo: www.agrociencia-panama.blogspot.com); (B)lesion on stem (photo: ICRISAT). |
Hosts
Many ornamental plants and leguminaceous weeds.
Geographic distribution
The disease was first reported from Brazil in 1927 and subsequently from several other countries like Puerto Rico, India, USA, Venezuela and Zambia (Nene et al. 1996).
Biology and transmission.
The conidia are cylindrical, broadly elliptical or irregular with rounded ends and 12-17 ´ 3.5-7.2 m in size. The setae are numerous, and are curved, septate and 100 ´ 3.5 m in size. Appressoria apparently analogous to chlamydospores and were observed after 2 months on PDA and steamed pigeonpea pods. The pathogen has been associated with seeds of pigeonpea and is considered as the quarantine important pathogen (Nirula 1980).
Detection/indexing methods at ICRISAT
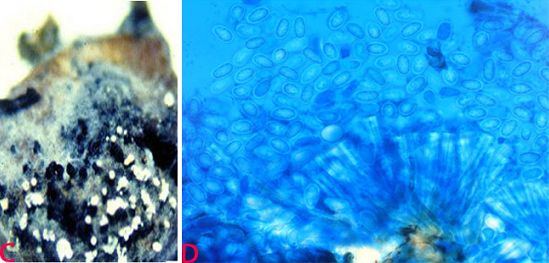
 Anthracnose of pigeonpea:(C)acervuli on seed and (D)conidia. (photos: ICRISAT). |
- Pre-export field inspection and blotter test are used.
Treatment/control
- Rejection of the infected seed samples.
Procedures followed in case of positive test at ICRISAT
- Rejection of the infected seed samples.
References and further reading
Nene YL, Sheila VK, Sharma SB. 1996. A world list of chickpea and pigeonpea pathogens. 5th edition. Patancheru, Andhra Pradesh, India: ICRISAT, pp27.
Nirula KK. 1980. Quarantine to seed exchange at the ICRISAT. Seed Pathology News 13: 11-12.
Reddy MV, Raju TN, Sharma SB, Nene YL, McDonald D. 1993. Handbook of pigeonpea diseases. Information bulletin no. 42. Patancheru, AP, 502324, India: International Crops Research Institute for the Semi-Arid Tropics. 64pp.
Tucker CM. 1927. Pigeonpea anthracnose. Journal of Agricultural Research 34: 589-596.
Scientific name
Alternaria alternata (Fries) Keissler.
Other scientific names
Alternaria fasciculate, Alternaria rugosa, Alternaria tenuis, Macrosporium fasciculatum, Torula alternate.
Importance
Medium.
Significance
In the late sown pre-rabbi pigeon pea, Alternaria blight has been identified as potential problem in some states of India (Reddy et al. 1993).
Symptoms
Alternaria alternata causes circular necrotic spots on leaves that develop quickly forming typical concentric rings. The lesions appear on aerial plant parts including pods. They cause severe blightening of the leaves and defoliation and drying of infected branches (Reddy et al. 1993).
 Alternaria alternata of pigeonpea: (A)leaf spot; (B)leaf blight (photos: ICRISAT). |
Hosts
Over 380 hosts have been recorded in the USDA Systematic Mycology and Microbiology Fungus-Host Distribution Database. It is an opportunistic pathogen on numerous hosts causing leaf spots, rots and blights on many plant parts. It can also cause upper respiratory tract infections in AIDS patients and asthma in people with sensitivity.
Geographic distribution
Alternaria blight is distributed in India, Kenya and Puerto-Rico (Reddy et al. 1993).
Biology and transmission
Mycelium of the pathogen is usually light olive green to brown. Hyphae are dark brown, thick, septate and branched. Conidiophores are pale brown to olive brown, 25-60 ´ 3-3.5 µm in size and straight or flexuous. Individual conidiophores arise directly from substrate forming bushy heads consisting of 4-8 large catenate conidia chains. Secondary conidiophores are generally short and 1-celled. Conidia are pale brown to light brown, obclavate to obpyriform or ellipsoid, short conical beak at the tip, or sometimes beakless. Surface of conidia is smooth to verruculose, 20-63 ´ 9-18 µm in size. Conidia are septate with several (1-5) vertical and 3-8 transverse septa. The fungus sporulates well under warm and humid conditions (Kannaiyan and Nene 1977). Alternaria alternata has been found to be internally seedborne and the infection was detected in all parts of the seed (Girish et al. 2007).
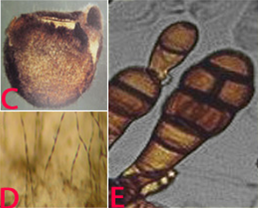 (C)fungal growth on seed; (D)conidia on seed and E. conidia (photos: ICRISAT). |
Detection/indexing methods at ICRISAT
- Pre-export field inspection and blotter test are used.
Treatment/control
- Seed treatment with the mixture of iprodione 25% + carbendazim 25% (1:1 by vol.) at 2 g a.i. kg-1 seed (Girish et al. 2007).
Procedures followed in case of positive test at ICRISAT
- Seed treatment with the mixture of benomyl + thiram (1:1) at 3 g kg-1 seed.
References and furter reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Girish AG, Sowjanya YV, Chakrabarty SK, Thakur RP. 2007. Studies on seedborne nature and management of Alternaria alternata and A. brassicae in pigeonpea. Indian Journal of Plant Protection 35: 128-130.
Kannaiyan J, Nene YL. 1977. Alternaria leaf spot of pigeonpea. Tropical Grain Legumes Bulletin 9:34.
Reddy MV, Raju TN, Sharma SB, Nene YL, McDonald D. 1993. Handbook of pigeonpea diseases. Information bulletin no. 42. Patancheru, AP, 502324, India: International Crops Research Institute for the Semi-Arid Tropics. 64pp.
Scientific name
Lasiodiplodia theobromae (pat.) Griffin & Maubl.
Other scientific names
Botryodiplodia ananassae, Botryodiplodia elasticae, Botryodiplodia theobromae, Botryodiplodia tubericola, Chaetodiplodia grisea, Diplodia ananassae, Diplodia theobromae, Lasiodiplodia nigra, Lasiodiplodia tubericola, Lasiodiplodiella triflorae, Macrophoma vestita.
Importance
Medium.
Significance
Lasiodiplodia theobromae affects a very wide range of crops, particularly at the postharvest stage. Infected seeds have reduced germination (Ellis et al. 1978).
Symptoms
Seed rotting and seedling blight.
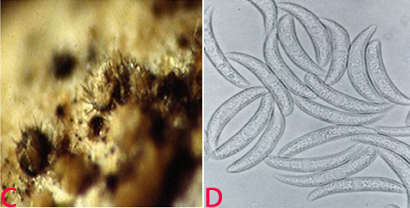
 Blight (Lasiodiplodia theobromae) of pigeonpea: (A)lesions on plant (photo: www.bspporg.uk.com); (B) pycnidia on seed. (Photo: ICRISAT) |
Hosts
Citrus aurantiifolia (lemon), Theobroma cacao (cocoa), Arachis hypogaea (groundnut), Gossypium (cotton), Musa (banana), Mangifera indica (mango), Zea mays (maize), Allium spp. (onions, garlic, leek, etc.), Ananas comosus (pineapple), Capsicum annuum (bell pepper), Dioscorea (yam), Cajanus cajan (pigeon pea), Glycine max (soyabean), Ipomoea batatas (sweet potato), Manihot esculenta (cassava), Nicotiana tabacum (tobacco), Oryza sativa (rice), Saccharum officinarum (sugarcane), Sorghum bicolor (Sorghum), Vigna unguiculata (cowpea) and Vitis vinifera (grapevine).
Geographic distribution
Lasiodiplodia theobromae is world wide in distribution especially in Africa, Asia, Western hemisphere and Oceania.
 (C)black and white conidial ooze on seed and (D)single celled immature conidia (photos: ICRISAT). |
Biology and transmission
Colonies of the pathogen on oatmeal agar are grayish or mouse grey to black, fluffy with abundant aerial mycelium, reverse fuscous black to black. Pycnidia appear on the infected leaves, stems and fruits. Pycnidia are immersed and thick-walled, and aggregated in clusters immersed in a stroma frequently up to 5 mm wide, erumpent, often with a distillate papillate ostiole (Punithalingam 1976). Conidiophores are hyaline, simple, sometimes septate, rarely branched, cylindrical, arising from the inner layers of cells lining the conidiomatal cavity. Conidia are initially aseptate, hyaline, granulose, subovoid to ellipsoid-oblong, thick-walled, base truncate. Mature conidia are one-septate, cinnamon to fawn, often longitudinally striate, 18-30 ´ 10-15 mm. Paraphyses are hyaline, cylindrical, sometimes septate, up to 50 mm long. Seedborne nature of L. theobromae was reported in about 29% of the seeds of pigeonpeas (Richardson 1990).
Detection/indexing methods at ICRISAT
- Pre-export field inspection and blotter method
Treatment/control
- Seed treatment with a mixture of benomyl + thiram (1:1) at 3 g kg-1 seed.
Procedures followed in case of positive test at ICRISAT
- Seed treatment with a mixture of benomyl + thiram (1:1) at 3 g kg-1 seed.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Ellis MA, Paschal EH, Ravalo EJ, Rosaria E. 1978. Effect of growing location on internally seed-borne fungi, seed germination and field emergence of pigeon pea in Puerto Rico. J. Agric. Univ. P.R 62:355-360.
Punithalingam E. 1976. Botryodiplodia theobromae, No. 519 In: Descriptions of Pathogenic Fungi and Bacteria. Wallingford, UK: CAB International.
Richardson MJ. 1990. An Annotated List of Seed-borne Diseases. 4th edn. Zurich, Switzerland: International Seed Testing Association.
Comments
- No comments found





Leave your comments
Post comment as a guest