Insects - pearl millet
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Scientific name
Plodia interpunctella Hubner.
Other scientific names
Not known
Importance
High
Significance
Indian meal moth is a common grain-feeding pest. It can infest a variety of products and is perhaps the most economically important insect pest of processed food. Infestations of P. interpunctella can cause direct product loss and indirect economic costs through pest control costs, quality losses, and consumer complaints (Phillips et al. 2000).
Symptoms
Plodia interpunctella is an external feeder. The larvae continuously spin a silken web both inside and on top of the grain surface, and feed within the web. The webbing contains larval excreta (frass) and exuvia (cast skins), and gives an unpleasant odor to the infested commodity. The infested commodity is sometimes covered on the surface with a thick mat of silken webbing (Mohandass et al. 2007).
Biology and transmission
Female moths lay between 60 and 400 eggs (Lyon 1991) on a grain surface, which are ordinarily smaller than 0.5 mm and not sticky. The eggs hatch in 2 to 14 days. The moth larvae are off-white with brown heads. When these larvae mature, they are usually about 12 mm long. The larval stage lasts from 2 to 41 weeks, depending on the temperature. Adult moths are 8–10 mm long with 16–20 mm wingspans. The outer half of their forewings is bronze, copper, or dark gray in color, while the upper half are yellowish-gray, with a dark band at the intersection between the two. The entire life cycle may range from 30 to 300 days (Mohandass et al. 2007).
 Indian Meal Moth (Plodia interpunctella) of finger millet (photo: ICRISAT). |
Hosts
Arachis hypogaea (groundnut), Oryza sativa (rice), Prunus (stone fruit), stored products (dried stored products), Triticum aestivum (wheat), Zea mays (maize), Avena sativa (oats), Corylus, Helianthus annuus (sunflower), Hordeum vulgare (barley), Juglans regia (Carpathian walnut), Pistacia vera (pistachio), Prunus dulcis (almond) and Theobroma cacao (cocoa).
Geographic distribution
Plodia interpunctella is cosmopolitan in distribution especially in warm climates. In cool temperate countries, it can survive in heated buildings.
Detection/indexing methods at ICRISAT
- The primary detection method is through pheromone-based trapping of males (Phillips et al. 2000). The pheromone commonly referred to as ‘‘ZETA’’ was one of the first commercial pheromones for stored-product insects, and the response of males to this pheromone has been well documented.
Treatment/control
- Fumigation with methyl bromide @ 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos at 3 g/kg-1 seed (Ghanekar et al. 1996; Toews et al. 2006).
Procedures followed in case of positive test at ICRISAT
- Infested samples are rejected in case of positive test.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43
Lyon WF. 2006. Ohio State University Extension Fact Sheet. Entomology. Indianmeal Moth Ohio State University. Retrieved on 2006-09-07.
Mohandass S, Arthur FH, Zhu KY, Throne JE. 2007. Biology, management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. Journal of Stored Products Research 43: 302-311.
Phillips TW, Berbert RC, Cuperus G.W. 2000. Post-harvest integrated pest management. In: Francis, F.J. (Ed.), Encyclopedia of Food Science and Technology. 2nd ed. Wiley Inc., New York, pp. 2690–2701.
Toews MD, Campbell JF, Arthur FH. 2006. Temporal dynamics and response to fogging or fumigation of stored-product Coleoptera in a grain processing facility. Journal of Stored Products Research 42:480-498.
Scientific name
Sitotroga cerealella Olivier.
Other scientific names
Tinea hordei
Importance
High
Significance
Grain losses due to grain moth infestation have been recorded up to 50%. High temperature and lack of storage hygiene are the major factors resulting in insect infestation (Seifelnasr 1992).
Symptoms
Infestation can begin in the fields. In storage, the infestation is confined to the upper layer of the grains. The larva bores into the grain and remains there until it emerges as an adult from round emergence holes. The infested grain is completely hallowed out and filled with larval excreta.
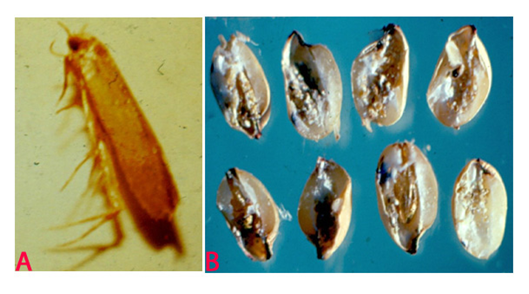 Angoumois Grain Moth (Sitotroga cerealella) of pearl millet: (A)adult and (B)infested grains (photos: ICRISAT). |
Hosts
Avena sativa (oats), Hordeum vulgare (barley), Oryza sativa (rice), Sorghum bicolor (Sorghum), Secale cereale (rye), Triticum aestivum (wheat), Triticum spelta (spelt) and Zea mays (maize).
Geographic distribution
It is cosmopolitan in distribution. It is one of the primary pests of the stored cereals grain, but is also known to attack maize, wheat, and barley in the storage godowns.
Biology
The female can lay up to 400 eggs that are deposited indiscriminately on or between the grains, on the panicles in the field, or in the storage. The egg is white and oval, but soon turns bright red and hatches within a week. The tiny larva crawls about searching for a comparatively weak spot through which it enters the grain, and feeds on the internal contents. The larval period is 2-3 weeks. The moth emerges within a week (Teetes et al. 1983).
Detection/indexing methods at ICRISAT
- Dry seed examination
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos at 3 g/kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Highly infested samples are rejected
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Seifelnasr YE.1992. Farmers’ perceptions on management practices of insect pests on stored sorghum in southwestern Ethiopia. Crop Protection 26: 1817-1825.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P. , India: International Crops Research Institute for Semi-Arid Tropics. 125pp.
Scientific name
Prostephanus truncatus Horn.
Other scientific names
Dinoderus truncatus
Importance
High
Significance
The grain damage by borer ranges from 19 to 38% and the mean dry weight loss between 5% and 20%. The quantity of grain dust produced weighed between 6 to 29 g kg-1 grains (Shires 1977; Mailafiya et al. 2008).
Symptoms
Adults attack stored seed/grain with intact sheaths by boring into the base of seed making neat round holes. As they tunnel from grain to grain large quantities of grain dust is generated. Adult females lay eggs in chambers bored at right angles to the main tunnels.
 Larger Grain Borer (Prostephanus truncatus) of finger millet (photo: www.agrsci.dk/). |
Hosts
Manihot esculenta (cassava), stored products (dried stored products), Zea mays (maize), Dioscorea (yam) and Triticum aestivum (wheat).
Geographic distribution
Prostephanus truncatus is indigenous in Central America, tropical South America, and the extreme south of the USA as a major pest. It is also distributed in Africa and Europe, and has restricted distribution in India (Farrell 2000).
Biology
The adult has the typical cylindrical bostrichid shape with body length of 3-4.5 mm. The declivity is flattened and steep, and has many small tubercles over its surface. The limits of the declivity, apically and laterally, are marked by a carina. The antennae are 10-segmented and have a loose three-segmented club; the 'stem' of the antenna is slender and clothed with long hairs and the apical club segment is as wide as, or wider than, the preceding segments. The larvae are white, fleshy and sparsely covered with hairs. They are parallel-sided, i.e. they do not taper. The legs are short and the head capsule is small relative to the size of the body (Farrell 2000).
Detection/indexing methods at ICRISAT
- Not detected.
Treatment/control
- Not suggested due to non-detection of the pest.
Procedures followed in case of positive test at iCRISAT
- Infested samples are rejected
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Farrell G. 2000. Dispersal, phenology and predicted abundance of the larger grain borer in different environments. African Crop Science Journal 8: 337–43.
Mailafiya DM, Ayertey JN, Cudjoe AR. 2008.Damage and weight loss potential of Prostephanus truncates Horn (Coleoptera: Bostrichidae) on sorghum grain: implication to cereal grain storage in sub-Saharan Africa. International Journal of pure and applied sciences 2: 28-35.
Shires SW. 1977. The ability of Prostephanus truncatus (Horn) to damage and breed on several food commodities. Journal of Stored Products Research13: 205-208.
Scientific name
Tribolium castaneum Herbst.
Other scientific names
Not known
Importance
High
Significance
Important in stored pearl millet grain in several countries.
Symptoms
Infestation by adult beetles can be readily observed by the tunnels they leave when they move through the flour and other granular food products. Damage is particularly serious in grains such as rice and wheat, which have either been dehusked or processed into other products. When infestation is severe, these products turn grayish-yellow and become moldy, with a pungent odor. Infestation may also be apparent by the appearance of adults on the surface of the grains (Teetes et al. 1983).
 Red Flour Beetle (Tribolium castaneum) of pearl millet: (A)adult and (B)damaged grains (photos: ICRISAT). |
Hosts
Arachis hypogaea (groundnut), Avena sativa (oats), Bertholletia excelsa (Brazil nut), Hordeum vulgare (barley), Juglans (walnuts), Culinaris (lentil), Oryza sativa (rice), Phaseolus (beans), Phaseolus lunatus (lima bean), Pisum sativum (pea), Prunus dulcis (almond), Secale cereale (rye), Triticum (wheat), Triticum spelta (spelt) and Zea mays (maize).
Geographic distribution
T. castaneum is cosmopolitan in distribution, found in infesting stored grain, seeds, flour, dried fruits and nuts (Teetes et al. 1983).
Biology and transmission
The adult is 2.3-4.4 mm long, rather flat, oblong and chestnut-brown (reddish-brown). It lays about 450 eggs in stored grain. The eggs are minute, cylindrical and white. The incubation period lasts for 5-12 days. The yellowish-white cylindrical grub is covered with fine hairs. The pupa is naked (without a cocoon), yellowish-white, becoming brown later and adults emerge in 3-7 days (Teetes et al. 1983).
Detection/indexing methods at ICRISAT
- X-ray radiography is used for suspected samples because it offers a non-destructive test of seed samples. Dry seed examination using magnifying lens to separate the infested seed.
Treatment/control
- Fumigation of the samples with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos at 3 g/kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Rejection of infested seed samples in case of positive test.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P., India: International Crops Research Institute for Semi-Arid Tropics. 125pp.
Comments
- No comments found





Leave your comments
Post comment as a guest