Fungi - faba bean
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
Ascochyta Blight, Leaf blight, Pod spot
Scientific names
Ascochyta fabae Gossen, Sheard, C.J. Beauch. & Morrall [anamorph], (1986): Didymella fabaeG.J. Jellis & Punith, (1991) [teleomorph].
Significance
Yield losses of 10-30% can occur in seasons favorable for the disease. Discoloration of seed can seriously reduce its market value.
Symptoms
Symptoms occur on leaves, stems and pods of infected plants and can be confused with the early stages of chocolate spot. On leaves, small, circular, dark-brown spots appear first. As the disease develops, they enlarge and turn light and then change to dark grey in color. They become irregular in shape, often zonate, and may coalesce to cover most of the leaf surface. Leaf tissue next to the lesions may become black and necrotic. Within the lesions, numerous pinhead- sized black fruiting bodies (pycnidia) of the fungus develop. These appear only under moist conditions and are often concentrically arranged on the stem; lesions are more elongated, sunken and darker than leaf lesions and are usually covered with scattered pycnidia. Stems may split and break at the point of infection causing plants to lodge. On pods, lesions are sunken and have pale centre and dark margins; they can be covered by numerous pycnidia. Well developed lesions can penetrate the pod and infect developing seeds causing them to be shrunken and discolored. Badly infected seeds have yellowish brown stains on the outer seed coat, which considerably reduces its market value.
Ascochyta blight can cause seed staining in pods close to maturity even when disease levels in the crop have been too low to warrant fungicide sprays. Faba bean seed that has greater than 25% seed coat discoloration can reduce the emergence of seed by 30%. Seed that has less than 5% seed coat discoloration will usually have normal levels of germination.
Hosts
Faba bean.
Geographic distribution
Africa (Egypt, Morocco); America (Argentina, Canada (NS); Asia (China, Syria, Israel, Japan, Korea, Turkey); Australasia (Australia, New Zealand); Europe (Britain, Czechoslovakia, Denmark, Germany, Italy, Jersey, Norway,Russia).
Biology and transmission
This fungal disease has an asexual and sexual stage; the asexual stage is most common. In this stage the fungus survives mainly on infected seed and on crop residues.
Spores of the fungus produced on crop residues can be carried onto new crops by wind. Infection can occur at any stage of plant growth, provided conditions are favorable. Moisture is essential for infection to occur.
During wet weather, the disease can spread further than in dry conditions because spores of the fungus are carried onto neighboring plants by wind and rain splash.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Malt Extract Agar
Treatment/control
Crop rotationc
- Since only Faba bean is susceptible to ascochyta blight caused by A. fabae inclusion of non-host crops in the rotation will bring about a reduction in the inoculum level.
- Faba beans should not be grown more frequently than every 3-4 years.
- Furthermore, new crops should not be grown near fields that were infested with blight in the previous year due to the risk of infection by air borne ascospores.
Tillage
- Under most conditions, deep ploughing will hasten decomposition of infected Faba bean straw and remove it as a source of inoculum. Studies conducted in the Pallouse Region in Washington State showed that the pathogen survived in naturally infected plant material for more than 2 years when situated on the soil surface, but lost its viability rapidly at soil depths of 10-40 cm. All infested crop residues and volunteer plants should therefore be destroyed by thorough tillage and proper crop rotation.
Fungicide application
- Growers should take special care to protect green and healthy foliage during pod filling, especially if blight symptoms appear and increase in severity. Tests with application of Bravo 500 at different crop stages have shown that spraying at early podding reduced pod damage and markedly increased seed yields in the moderately resistant cultivars. Foliar fungicide applications may not prove cost effective when disease pressure is low. Thiram-based fungicides are registered for the treatment of grey mould infected seed. While seed treatment can improve establishment, it does not provide any protection from air-borne infection later in the crop.
Procedure followed at the centers in case of positive test
- Destroying seed for intensive spots, and seed treatment for no visible spots
References and further reading
Ascochyta Leaf and Pod Spot of Faba Bean [online]
CABI. Ascochyta fabae. [Distribution map] [online].
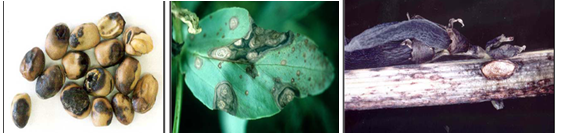 Ascochyta blight (photos:dpi.vic.gov.au) |
Scientific name
Botrytis fabae Sardina
Other scientific name
Botryotinia fabaeWu & Lu (1991).
Significance
Loss depends on the severity of infection, the time at which infection occurred, and the amount of spring rainfall. In unprotected crops, the disease can reduce yields by at least 30-50% under conditions favorable for disease development. In addition, seed from badly affected plants may have a reddish-brown discoloration, which lowers its market value.
Symptoms
These are varied, and range from small spots on the leaves to complete blackening of the entire plant. Leaves are the main part of the plant affected, but under favorable conditions for the disease it also spreads to stems, flowers and pods. Two stages of the disease are usually recognized. First, a non-aggressive phase, when discrete reddish-brown spots are "peppered" over the leaves and stems, and then an aggressive phase, when spots darken in color and coalesce to form larger grey-brown target spots that may eventually cover the entire plant. Small black sclerotia may sometimes be found inside the stems of badly diseased plants.
Red-legged earth mite (RLEM) damage can be mistaken for chocolate spot. This starts as silvery patches which become red-brown, similar in color to chocolate spot but form large irregularly-shaped areas. Red-legged earth mite damage usually occurs during the seedling stage and on the lower leaves.
Factors such as phosphorus or potassium deficiency, water logging and excessive weed burdens which reduce crop vigor may make plants more susceptible to the development of chocolate spot. Leaves which have been damaged by insect attack or in wheel tracks are also more susceptible.
Hosts
Vicia spp. especially Vicia faba (field or broad bean); also reported from Phaseolus beans, pea and lentil.
Geographic distribution
Europe, Middle East, N.E. and S. Africa, S.E. Asia, Australasia, S. America. More recent records are from Korea, India, S. Australia, Canada and Norway.
Biology and transmission
Chocolate spot, caused by Botrytis fabae and Botrytis cinerea can survive either as sclerotia in the soil or on crop debris, in infected seed, or on self-sown volunteer plants. In hitherto unaffected areas the disease often becomes established by the sowing of infected seed. In subsequent years, the initial infection usually occurs when spores formed on old bean trash are carried by wind into new crops. These spores may move long distances. Once the disease becomes established it rapidly spreads within a crop, and within 4-5 days of infection spores can be formed on infected tissue and initiate secondary spread of the disease. The fungus is most aggressive under cool, humid conditions, particularly at flowering time. Aggressive development of stem infection late in the season can cause the crop to lodge.
Air-borne conidia are readily produced on dead bean foliage during wet weather. The fungus perennates in bean debris and as sclerotia in the soil and is also seed-borne.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Agra Plate Test
Treatment/control
- Use of clean seed is advocated. Seed treatment with fungicides such as benomyl (0.2-0.1%) chlorothalonil (0.2-0.3%) or thiabendazol (0.1-0.2%) is recommended to control the seed- borne infection. Practice a 2-3 year crop rotation along with deep plowing to reduce soil-borne inoculum.
- Foliar spray using chlorothalonil (2-3 L\ha) as single application at early bloom to early pod set provides the best protection and increases seed yield.
Procedure followed at the centers in case of positive test
- Destroying serious infected seed, and seed treatment for light infection.
References and further reading
CABI. Botrytis fabae. [Descriptions of Fungi and Bacteria]. [online]
Richardson H, Horsham DPI. 2009. Chocolate Spot of Faba Bean. [online]
 Chocolate spot (photos:dpi.vic.gov.au) |
Botrytis grey mold of faba bean
Scientific name
Botrytis cinerea Pers. 1794
Other scientific name
Botryotinia fuckeliana (de Bary) Whetzel
Significance
The disease is capable of causing serious yield losses in years when spring rainfall is high and/or there are prolonged wet periods especially if it is founded with B. fabae.
Symptoms
All aboveground plant parts of Faba bean can be affected by botrytis grey mould. Depending on the location of the crop, symptoms may initially appear either on flowers and pods, or lower in the crop canopy. The most damaging symptoms become apparent after the crop has reached canopy closure and a humid microclimate is produced under the crop canopy. The disease appears first as discrete cream colored lesions on lower leaves. These enlarge and coalesce to infect whole leaflets which later senesce and fall to the ground. Unlike ascochyta blight, no small, black fruiting bodies (called pycnidia) can be seen within the lesions. If conditions remain conducive for disease, that is warm and wet under the crop canopy for at least 4 days, infection can spread to the lower stems. These lesions will girdle the stem and become covered with a furry layer of grey mould, eventually causing stem death and whole plant death, often occur before the onset of flowering and pod fill. Infection will continue to spread resulting in patches of dead plants within crops. Pods which become infected will be covered in a grey moldy growth, rot, and turn brown when dried out.
Hosts
Botrytis cinerea has a broad crop host range collectively, including faba bean, chickpea, field pea, lupin and pasture legumes such as lucerne and clover. Other host species include a wide range of ornamental and horticultural crops.
Geographic distribution
Cosmopolitan.
Biology and transmission
The fungal pathogens Botrytis cinerea that cause botrytis grey mould can survive as several forms, these include in infected seed, sclerotia in the soil, in old infected trash, and on alternate host plants. Sowing seed that is infected by the botrytis grey mould pathogens can give rise to infected seedlings and the appearance of seedling blight symptoms, which can reduce seedling survival and reduce crop establishment. Old infected trash is an important source of fungal inoculum. Spores are produced on old trash and are carried by the wind into new crops where infection can occur.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Agar Plate Test
Treatment/control
- Use of clean seed is advocated. Seed treatment with fungicides such as benomyl (0.2-0.1%) chlorothalonil (0.2-0.3%) or thiabendazol (0.1-0.2%) is recommended to control the seed- borne infection. Practice a 2-3 year crop rotation along with deep plowing to reduce soil-borne inoculum.
- Foliar spray using chlorothalonil (2-3 L\ha) as single application at early bloom to early pod set provides the best protection and increases seed yield.
Procedure in case of positive test
- Seed treatment
References and further reading
Scientific name
Colletotrichum lindemuthianum (Sacc. & Magnus) Briosi & Cavara.
Other scientific name
Colletotrichum truncatum (Schwein.) Andrus & Moore.
Significance
Not Significant
Symptoms
Leaf lesions and premature leaf drop: In most faba bean crops, the first symptoms of anthracnose appear before flowering, when the plants have 8 to 12 nodes on the main stem. This is also the time when the first tendrils form, and approximately a week before flowers start to open. If there is a large amount of inoculum in the field the first symptoms may appear earlier. Tan coloured lesions of variable size develop on the lower leaflets and the most severely affected leaflets die and drop to the ground. This premature leaf drop indicates that anthracnose may become a problem, and fungicide application should therefore be considered.
Stem lesions: Lesions on stems develop soon after the appearance of leaf lesions, primarily at the base of the plant. Stem lesions may be small, brownish with a black border, or larger, stretching along the stem. As the season progresses, more and more lesions develop at the stem base, as well as on the upper part of the stems, and many stems are girdled.
Wilt: Anthracnose causes defoliation and stem girdling, which inhibits utilization of water and nutrients, and causes the faba bean plants to wilt. As a result, large areas of brown and dying plants can be found in the field.
Hosts
The fungus Colletotrichum truncatum causes anthracnose in lentil and only attacks plant species belonging to the Lens and Vicia family, such as fababean and wild vetches. This means that a few weedy species in Canada can harbor the disease, but other crops, except fababean, are not at risk.
Geographic distribution
It is reported from Bangladish, Canada, Ethiopia, Morocco, and Syria. It is economically important only in Canada.
Biology and transmission
Small, pinhead sized fungal structures (microsclerotia) form on the infected plant tissue. They may be seen with the unaided eye in the centre of stem lesions or more easily with a hand lens (10-15 x magnification). Each microsclerotia consists of a few hundred cells with thick, black cell walls that protect the fungus from colonization by other microorganisms. Microsclerotia enable the fungus to survive between faba bean crops either on the plant debris or free in the soil. They remain viable longer when buried in the soil by tillage than left exposed to weather extremes on the soil surface.
Detection/indexing method at ICARDA
- Agar Plate Test
Treatment/control
- Use either certified seed, approved seed, or seed known to have a long disease-free history. The use of disease-free seed is the most important control measure.
- Do not plant beans for at least two years in land that has carried an infected crop.
- Remove diseased plants, where practical, to help check the spread of disease.
- Avoid cultivating and harvesting an affected crop when wet to prevent the spread of spores.
- Do not pack lightly diseased pods as anthracnose can develop during transport
- Bravo 500 (50% cholorothalonil, Zeneca Agro). The recommended rate is 0.8 - 1.6 L/acre (2.0-4.0 L/hectare) with a maximum of two applications in a season. The water rate is 90-640 L/acre (220-1600 L/hectare). Bravo must be present on the plant surface prior to the onset of fungal infection. It sticks well to the plant surface and resists removal by rain. A second application 10-14 days later may be necessary under wet weather conditions and to protect new growth. Fungal resistance to cholorothalonil has not been detected.
Procedure followed in case of positive test
- Seed treatment
References and further reading
Minchinton E, Knoxfield. 1999. Anthracnose of beans. [online]
North Dakota State UNiversity. Anthracnose Disease In Lentils. [online]
 Anthracnose (photo:paridss.usask.ca) |
Phoma blight, Spring black stem, Leaf spot
Scientific name
Phoma medicaginis var. medicaginis Malbr. & Roum, 1886.
Other scientific name
Phoma medicaginis
Significance
Not Significant
Symptoms
Small, dark brown to black dots on leaves, petioles and stems;
Leaf spots enlarge, coalesce forming irregular blotches;
Infected leaves turn yellow, wither and drop;
Black tissue may appear near base of stems;
Crown and root rot may occur; and
Infected seed pods and seed may discolour and shrivel.
Hosts
All legumes and specially alfalfa.
Geographic distribution
Cosmopolitan
Biology and transmission
Favoured by cool and wet conditions; overwinters on dead stems and leaves or in crowns and roots; spores released during periods of wet cool weather; spores spread by rain splash, wind blown or carried by insects; and new shoots exposed through infected residue.
Detection/indexing method at ICARDA
- PDA
Treatment/control
- Use certified seed; use disease resistant cultivars; crop rotation; and crop rotation; and spring burning when severe.
- Application of benomyl seed treatments (0.1 and 0.5% w/w) resulted in only a 4-5 week delay in the onset of Phoma black stem symptoms.
Procedure followed at the centers in case of positive test
- Seed treatment
References
Manitoba Agriculture, Food and Rural Initiatives. Management of Diseases of Alfalfa Seed. [online]
Fusarium wilt, Fusarium root rot
Scientific names
Fusarium oxysporum, F. moniliforme, F. equiseti, F. tricinctum and F. solani. [anamorphs]
Other scientific names
Gibberella avenacea, Gibberella moniliformis, Gibberella intricans, Gibberella tricincta, Gibberella zeae.[Teleomorphs]
Significance
The diseases cause up to 100% yield loss under heavy infestation, depending on relative humidity, soil moisture, and soil temperature.
Symptoms
The disease appears in the field in patches at both seedling and adult stages. Seedling wilt characterized by sudden drooping followed by drying of leaves and whole seedling, and apparently healthy looking roots with reduced proliferation.
The adult wilt symptoms first appear at flowering to late pod-filling stage and are characterized by sudden drooping of top leaflets of the affected plant, leaflets closure without premature shedding, dull green foliage color followed by wilting of whole plant or individual branches, apparently healthy looking roots system with a slight reduction in the lateral roots which are as difficult to pull out as the healthy plant, and no internal discoloration of the vascular system in most cases.

Fusarium |
Hosts
Widespread.
Geographic distribution
Worldwide.
Biology and transmission
If a field becomes infested with either of the wilt fungi, it remains so indefinitely. The causal fungi can be carried from one field to another on farm equipment, on lentil refuse, and in wind- or waterborne soil. They may be introduced into new areas on the seed. Once introduced into a field it may take two years or more for the fungi to increase in numbers where an appreciable amount of disease is evident. A soil temperature of (23° to 27°C) is most favorable for Fusarium or true wilt, and a slightly higher optimum temperature (27°C) for near wilt. Race 5 of the near wilt fungus, not found in Illinois, infects at lower temperatures. The wilt-producing fungi are soil inhabitants that penetrate the lentil plant through the root hairs and fibrous roots. They grow upward through the stem, often well into the upper branches, in the water conducting tissue (xylem). This process interferes with the passage of water from the roots to the stems, leaves, and pods resulting in yellowing, dwarfing, and wilting of plants. The Fusarium fungi do not reproduce on living plants but produce large numbers of microscopic spores (microconidia, macroconidia, and chlamydospores) in and on dead stems and roots. The spores are splashed or blown about within fields. The spores germinate and the resulting hyphae penetrate the host. The fungi survive in soil for 10 years or longer, in the absence of a lentil crop, as thick-walled chlamydospores. Survival is related to the association of the Fusarium fungi with the roots of nonhost crops. The fungi are also capable of infecting seeds.
Detection/indexing method at ICARDA
- Blotter Test
Treatment/control
- Choose resistant varieties when available. Remove stricken growth and sterilize clippers (one part bleach to 4 parts water) between cuts.
- Control garden insects, such as cucumber beetles, which are known to spread the disease.
- Remove all weeds from the garden (many weed species host the disease). The biological fungicide Mycostop will control wilt caused by Fusarium. If the disease persists, it is best to remove the entire plant and solarize the soil before planting again.
- To solarize the soil, you must leave a clear plastic tarp on the soil surface for 4-6 weeks during the hottest part of the year. Soil solarization will reduce or eliminate many soil inhabiting pests, including nematodes, fungi, insects, weeds and weed seeds.
Procedure in case of positive test
- Rotation, seed treatment
References and further reading
Economic Plants and their Diseases, Pests and Weeds. Diseases: Fusarium Root Rot and Fusarium Wilt. [online]
PAN Germany, OISAT. Fusarium wilt. [online]
Planet Natural. Fusarium wilt. [online]
Stem rot, Sclerotinia crown rot and root
Scientific name
Sclerotinia sclerotiorum (Lib.) de Bary, (1884)
Other scientific name
Hymenoscyphus sclerotiorum(Lib.) W. Phillips, (1887).
Significance
Sclerotinia sclerotiorum infects nearly 400 plant species and causes economic damage to a wide range of crops.
Symptoms
Signs and symptoms caused by S. sclerotiorum vary depending on the host. However, the most obvious sign is the appearance of white fluffy mycelial growth that will later produce sclerotia. The fungus infects and produces mycelium at the base of the plant that will eventually move up the stem causing it to rot.
Sclerotinia sclerotiorum on seedlings (collar rot) produces small black-brown lesions at the base of the stem. These will eventually cause rapid destruction of the seedlings as the fungus moves up the stem and into the leaves. Fruits such as cucumber, squash, and eggplant are also susceptible to S. sclerotiorum. In this case the fungus causes a wet rot that will eventually engulf the entire fruit. White mycelium can be seen both externally and within the infected fruit.
 Sclerotinia disease (photo: www.igzev.de) |
Hosts
Sclerotinia sclerotiorum is among the most nonspecific, omnivorous, and successful of plant pathogens. Plants susceptible to this pathogen encompass 64 families, 225 genera, and 361 species.
Geographic distribution
Sclerotinia sclerotiorum has a world-wide distribution; however it is most prevalent in cool moist regions.
Biology and transmission
Approximately 90% of the life cycle of Sclerotinia species is spent in soil as sclerotia. At certain times of the year, depending on the inherent nature of the fungus and various environmental factors, the sclerotia germinate and form either mycelium which can infect a host, or an apothecium. Infection of host plants by mycelium can occur at or beneath the soil-line. Sclerotia germinate in the presence of exogenous nutrients and produce hyphae which invade nonliving organic matter, forming mycelium which then infects living host tissues. Penetration of the host cuticle is achieved by mechanical pressure. Mycelium infection is unlikely to infect plants located more than 2 cm from a sclerotium.
Apothecium produced by Sclerotium releases ascospores that disseminate by air. Generally, 10-20 C is the optimum temperature range for carpogenic germination. Under favorable conditions, including adequate moisture, ascospores germinate within 3-6 hours of release. In one study, apothecia collected from the field discharged ascospores continuously for seven days in the laboratory. Ascospores infect nonliving host tissues, germinate, and inundate the nonliving plant part with mycelium. Then, the fungus invades healthy plant tissues with mycelium. After the plant or plant part dies, sclerotia are formed either on or in the plant. The sclerotia return to the soil for a "resting" period (which can be weeks or years) before they become active, which requires the appropriate environmental conditions.
Sclerotia are the structures which allow species of Sclerotinia to survive for long periods of time under adverse conditions. The black, melanized rind appears to act as a protectant from invasion by microorganisms. Soil temperatures, pH, and moisture appear to have little direct effect on their survival, though the combination of high temperatures and high moisture appears to encourage the degradation of sclerotia near the soil surface.
Air-borne ascospores are the most important means of spread. Mycelium from sclerotia can also cause infection, but usually this mode of infection stays within an area. Moving contaminated soil (on farm equipment, shoes, infected seedlings) and fertilizing with manure from animals fed infected plant debris, are two common ways of spreading sclerotia or mycelium from place to place. Irrigation also has been shown to be involved in the spread of Sclerotia spp. from field to field. Irrigation transported sclerotia remained viable for at least 10-21 days in flowing water. In addition, seed may be an infective source, either from contaminating sclerotia or internal mycelium. However, support for this method of transmission is questionable.
Detection/indexing method at ICARDA
- PDA , blotter test
Treatment/control
- There are essentially three methods of controlling Sclerotinia sclerotiorum; chemical, cultural, and biological control. Chemical and cultural control is most widely used, while biological control is used to a lesser degree. The only chemical control that is employed to control S. sclerotiorum is fumigation. The soil is fumigated to reduce the amount of inoculum present. Cultural practices are often used to prevent the spread of the pathogen from one host to another by killing the pathogen or by providing unfavorable conditions for the pathogen to develop. Sanitation, proper ventilation, and adequate spacing of plants are all effective cultural practices used by growers in controlling S. sclerotiorum. Although biological control is used less extensively than chemical and cultural methods, some biological control agents have been labelled. Many antagonistic fungi such as Coniothyrium minitans, Gliocladium roseum, Gliocladium virens, Sporodesmium sclerotivorum, and Trichoderma viride have been shown to provide effective control of S. sclerotiorum. These mycoparasitic fungi inhibit the growth of S. sclerotiorum by destroying existing sclerotia while inhibiting the formation of new sclerotia. All three methods for the control of S. sclerotiorum are important to long term health and viability of the plants affected by this pathogen. Addition of compost and organic fertilizers can decrease disease levels. Fungicides such as methyl thiophanate, PCNB (pentachloronitrobenzene) and chlorothalonil are also effective. Consult label directions and carefully follow instructions for application.
- Removing the debris after harvest will reduce the amount of inoculum in the soil. In some cases, alternation with a nonsusceptible crop also reduces inoculum level.
Procedure in case of positive test
- Rotation and seed treatments
References and further reading
http://www.extento.hawaii.edu/Kbase/Crop/Type/s_scler.htm
http://www.cals.ncsu.edu/course/pp728/Sclerotinia/S_sclerotiorum.html
http://www.igzev.de/englisch/projekte.php?abteilung=4
Scientific names
Stemphylium botryosum Rotem,Y. Cohen & I. Wahl, (1966) [Anamorph]:
Pleospora herbarum(Pers.) Rabenh., (1854) [Teleomorph]
Significance
It is reported to be seedborne
Symptoms
Spot-causing fungal disease occurring in leaf. The lesions are at first water-soaked small spots and then become blackish brown lesion divided by leaf veins. The lesions often extend from the leaf rim, fuse with the adjacent lesion, and become large. The leaf shrinks and withers in results. The diseased leaf defoliates at the early stage. The species of the causal organism is same with leaf spot fungi of alfalfa, but the pathogenicity is differentiated.
Hosts
On a wide range of hosts including apple, ash, broad bean, clover, endive, gladiolus, gramineae, lettuce, lupin, muskmelon, onion, Onobrychis, Medicago sativa, mangold, tomato, Trifolium, Vicia.
Geographic distribution
Worldwide; very common in temperate and sub-tropical regions.
Biology and transmission
Survival: The fungi are carried-over on infected plant debris (leaves and stems), seed and in the soil.
Environmental conditions: Stemphylium leaf spot may develop under warm moist conditions.
Dispersal: Spores of these fungi are spread by wind and rain splash. Generally air-borne as ascospores or conidia. These penetrate the leaf or petiole via stomata. May also occur on seeds and in soil.
Detection/indexing method at ICARDA
- Blotter test, agar plate method
Treatment/control
- Seed treatment. These diseases are usually not a problem after the end of the rainy season except in Valleys where they can cause damage up to harvest under conditions of high humidity and heavy night time dew. (ICARDA).
Procedure followed in case of positive test
- Seed treatment
References and further reading
CABI. Pleospora herbarum. [Descriptions of Fungi and Bacteria]. [online].
Clarke R, Knoxfield. 1999. Diseases of lucerne - 2: Fungal leaf diseases. Agriculture Notes. [online].
Scientific names
Rhizoctonia solaniJ.G. Kühn 1858 [Anamorph]
Thanatephorus cucumeris(A.B. Frank) Donk 1956 [Teleomorph].
Significance
Not Significant.
Symptoms
R. solani primarily attacks below ground plant parts such as the seeds, hypocotyls, and roots, but is also capable of infecting above ground plant parts (e.g. pods, fruits, leaves and stems). The most common symptom of Rhizoctonia disease is referred to as "damping-off" characterized by non germination of severely infected seed whereas infected seedlings can be killed either before or after they emerge from the soil. Infected seedlings not killed by the fungus often have cankers, which are reddish-brown lesions on stems and roots. In addition to attacking below ground plant parts, the fungus will occasionally infect fruit and leaf tissue located near or on the soil surface. This type of disease often occurs because the mycelium and/or sclerotia of the fungus are close to or splashed on the plant tissue.
Although most Rhizoctonia diseases are initiated by mycelium and/or sclerotia, several important diseases of beans, result from basidiospore infection. These basidiospores also serve as a source for rapid and long distance dispersal of the fungus. The basidiospores germinate to produce hyphae that infect leaves during periods of high relative humidity and periods of extended wet weather. Under these conditions, basidiospores can often be observed on the base of stems near the soil surface or on the underside of leaves in the plant canopy.
The pathogen is transported in infested soil or through movement of diseased plants or bean pods. Potential for seed-borne inoculum also exists.
Although basidiospores are wind-borne, their role in initiating disease has not been considered important except for foliar diseases (web blight) in high humidity.
Hosts
R. solani is a very common soilborne pathogen with a great diversity of host plants.
Geographic distribution
Common throughout the world.
Biology and transmission
Rhizoctonia solani produces thread-like growth called hyphae ; large masses of hyphae are referred to as mycelium. The hyphae of Rhizoctonia solani have the following characteristics, some shade of brown, a special type of cross wall within the hyphae, called a dolipore septum, each cell is multinucleate (has many nuclei) rather than binucleate, branches that are produced at right angles, no asexual spores are formed by the mycelium.
In general, the growth rate of Rhizoctonia solani is very rapid and a typical isolate can grow across a 90 mm petri plate in three days. Small, oval cells produced in branched chains or clusters are formed. These are called monilioid cells and have slightly thicker walls than the mycelium. Large aggregates of these cells are called sclerotia which are black to brown and 3 to 5 mm long.
The hyphae of Rhizoctonia solani have many nuclei (commonly 4 to 8) per cell. This distinguishes it from similar fungi that have only 2 nuclei per cell. Those fungi with hyphal characteristics similar to Rhizoctonia solani, but with only 2 nuclei per cell, are called binucleate types and are generally non-pathogenic. These Rhizoctonia solani-like fungi are saprophytic, do not cause disease, and feed on dead organic matter.
Following invasion of the host by Rhizoctonia solani, sexual spores are formed on specialized structures called basidia. Four spores are produced on each basidium. Basidia are formed when the environment is moist and sufficient growth of the fungus has occurred. Basidiospores are wind-dispersed and germinate with moisture. Each basidiospore has a single nucleus. The hyphae produced by germinating spores will fuse (anastamose), forming new hyphae with a mixture of different types of nuclei.
Observations of basidiospores on diseased host tissue are not common. Several methods have been developed to produce basidiospores in pure culture but it remains difficult.
In recent years, the recognition of different anastomosis groups has helped to elucidate the complexity of this large fungal group. Having no asexual spores and only the few morphological characteristics to define "Rhizoctonia solani isolates", many fungi were placed into this species. However, it is now recognized that Rhizoctonia solani has multinucleate cells and other related fungi (with 2 nuclei per cell), the binucleates, do not belong in that species. Further, when isolates are paired, only related mycelium will fuse and thus anastomosis groups have been defined by a set of tester isolates. A set for Rhizoctonia solani isolates and another set for binucleate Rhizoctonia solani-like fungi are also available.
Detection/indexing method at ICARDA
- Agar Plate Test
Treatment/control
- Obtain high quality seeds and avoid seeds that may be contaminated with the pathogen. In fields known to have Rhizoctonia solani, prepare planting areas to increase drainage and prevent water accumulation. Set plants with adequate spacing to avoid crowding and formation of high humidity in the foliage.
- Addition of compost and organic fertilizers can decrease disease levels. Fungicides such as methyl thiophanate, PCNB (pentachloronitrobenzene) and chlorothalonil are also effective. Consult label directions and carefully follow instructions for application.
- Removing the debris after harvest will reduce the amount of inoculum in the soil. In some cases, alternation with a nonsusceptible crop also reduces inoculum level.
Procedure followed in case of positive test
- Rotation, seed treatment
References and further reading
http://www.extento.hawaii.edu/Kbase/Crop/Type/r_solani.htm#MANAGEMENT
http://www.cals.ncsu.edu/course/pp728/Rhizoctonia/Rhizoctonia.html
 Rhizoctonia root disease (photos:www.cals.ncsu.edu) |
Comments
- No comments found





Leave your comments
Post comment as a guest