Viruses - lentil
Contributors to this page: ICARDA, Aleppo, Syria (Siham Asaad, Abdulrahman Moukahal).
|
Contents: |
Scientific name
Bean yellow mosaic virus (BYMV)
Other scientific names
Bean virus 2 (BV-2), canna mosaic virus, gladiolus mosaic virus, gloriosa stripe mosaic virus
Significance
During our pulse surveys from 2000-2005, BYMV infections were uncommon and found in less than 1% of surveyed crops. However, BYMV was sometimes found in faba bean crops and occasionally in peas with within crop virus incidences of 1-15% and up to 7% respectively. In NSW, a small survey showed that faba bean crops had an average within crop incidence of BYMV of 26% of plants (ranging from 1-63%) (van Leur et al. 2002). Field surveys in 1998-1999 showed that some faba bean and field pea crops were infected with BYMV and the within crop virus incidences were 1-31% and 1-11% respectively. Plot trials showed that lupins infected with the necrotic strain of BYMV can have grain yields reduced by 95%.
Symptoms
Leaves of infected plants show mild mosaic followed by narrowing. New growth from leaf axils shows leaves that are narrow, elongated and light green. Early infections adversely affect plant growth and yield; there may be reduction in leaf size and stunting. Infected plants produce very little seed.
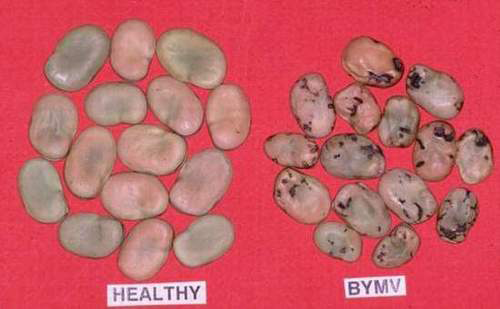
 Bean yellow mosaic (photo: ICARDA) |
Hosts
Wide host range. BYMV is reported to infect nearly 200 species in 14 families.
Legume hosts include lentils, chickpeas, beans, faba beans, green beans, peas, soybeans, peanuts, lupins, lathyrus, lucerne, vetch, clover, and various pasture hosts. The virus also infects horticultural and ornamental plants, such as gladiolus species.
Geographic distribution
Cosmopolitan
Biology and transmission
BYMV is transmitted by more than 50 aphid species in a non-persistent manner: the main species are Acyrthosiphon pisum, Aphis fabae, A. gossypii, Aulacorthum solani, Brevicoryne brassicae, Myzus persicae and Rhopalosiphum maidis. During our surveys in Victoria we have found the following BYMV vectors in pulse crops: Acyrthosiphon kondoi, Aphis craccivora, Aulacorthum solani, Brevicoryne brassicae and Myzus persicae. In WA Acyrthosiphon kondoi, Aphis craccivora, Myzus persicae, Brachycaudus rumexicolens, Lipaphis erysimi, Rhopalosiphum maidis, R. padi, Sitodion miscanthi and Therioaphis trifolii have been reported as BYMV vectors.
The virus is also transmitted through seed of most temperate pulses, including faba beans, field peas, lentils, and lupins and through seed of a number of forage legumes and clovers. In Victoria, we found 18% of lentil seed lots tested had BYMV infections of 0.1-0.9%. In WA, the following BYMV seed transmission is reported: yellow and white lupins 3-6%, field peas 0.3-0.8%, faba beans 0.4%, lathyrus 0.1-0.2% and vetch 0.5%.
Seed transmission of BYMV in medics and clovers has also been reported in WA as follows: Melilotus indica (0.5%), Medicago polymorpha (0.9%), M. truncatula (0.3%), M. indica (1%), Trifolium arvense (0.1%), T. campestre (0.2%) and T. glomeratum (0.05%).
Detection/indexing methods used at ICARDA
- Tissue blot immunoassay (TBIA)
- Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be one of the main sources of BYMV, therefore sowing virus tested seed is recommended and commercial seed tests are available. BYMV causes heavy losses in narrow leafed lupins, so only virus tested seed is recommended for sowing. Virus resistant lupin varieties are now available. BYMV-infected pastures are another major source of the virus, which is then spread to crops by aphids. Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses such as BYMV. Pulse crops should be sown away from legume pastures to minimise the spread of BYMV. The spread of virus can also be reduced by controlling weed hosts from in and around paddocks.
Procedures followed in case of positive test at ICARDA
- The seed lot is destroyed.
References and further reading
Aftab M, Freeman A. 2006. Temperate pulse viruses: Bean yellow mosaic virus (BYMV). Agriculture note AG1266. Melbourne: Department of Primary Industries, Victoria, Australia.
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Bean yellow mosaic. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lentil.htm#Lent7.html. Date accessed 13 April 2010
 |
 |
|
|
Bean yellow mosaic virus (BYMV) (photos: Department of Primary Industries, Victoria, Australia) |
||
Scientific name
Broad bean stain virus (BBSV)
Other scientific names
Broad bean F1 virus, broad bean Evesham stain virus
Significance
In a study of 19 lentil genotypes in Syria, yield loss due to infection varied between 14% and 61% (Makkouk and Kumari 1990).
Symptoms
The disease is characterized by a mild mottling on the leaves, although this is not easy to recognize due to the small size of lentil leaf.
Affected plants are reduced in growth, which is easily recognized, especially in comparison with healthy plants. Yield of affected plants is also reduced.
Seed from infected plants occasionally show dark staining.
 Healthy lentil seed (right) and seed with BBSV symptoms (left) which include dark staining and distortion.(photo: PaDIL, ICARDA). |
Hosts
The virus is restricted to legumes. In addition to lentil, it also naturally affects faba bean (or broad bean), dry pea and vetch.
Geographic distribution
Europe, Middle East, North Africa, West Asia
Biology and transmission
BBSV is transmitted by insect vectors, the weevilsApion vorax and Sitona spp. It is also transmitted through sap and seed. In 19 lentil genotypes studied in Syria seed transmission rates varied between 0.2% and 32.4% (Makkouk and Kumari 1990).
Detection/indexing methods used at ICARDA
- Tissue blot immunoassay (TBIA)
- Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- It is important to avoid the use of infected seed. Lentil should be planted away from other legume hosts and infected plants should be promptly removed to prevent spread of the disease.
- As BBSV is transmitted by weevils, seed may be treated with Promet (12 ml/kg seed) to help control the vector and hence disease spread.
Procedures followed in case of positive test at ICARDA
- The seed lot is destroyed.
References and further reading
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Broad bean stain. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lent7.Html#_Broad. Date accessed 13 April 2010
Gibbs AJ, Smith HG. 1970. Broad bean stain virus. Descriptions of plant viruses 29. Association of Applied Biologists. [online] Available from URL: http://www.dpvweb.net/dpv/showdpv.php?dpvno=029. Date accessed 13 April 2010
Makkouk KM, Kumari SG. 1990. Variability among 19 lentil genotypes in seed transmission rates and yield loss induced by broad bean stain virus infection. LENS Newsletter 17(2): 31-33.
Plants and Diseases Image Library (PaDIL). Plant biosecurity toolbox: Broad bean stain virus (BBSV). [online] Available from URL: http://www.padil.gov.au/pbt/index.php?q=node/20&pbtID=154. Date accessed 13 April 2010
Scientific Name
Pea Seed-borne Mosaic Virus (PSbMV)
Other scientific names
Pea fizzle top virus, pea leaf rolling virus, pea leaf roll mosaic virus, pea leaf rolling mosaic virus.
Significance
PSBMV is of economic importance in pea, faba bean and lathyrus, mainly due to its effect on seed quality. It has been mistakenly considered to be a minor disease because it often causes only minor yield loss and mild symptoms, However, at the International Centre for Agriculture in the Dry Areas (ICARDA), Syria, glasshouse studies on yield losses due to PSBMV in chickpea, faba bean, lentil and pea showed losses of 66%, 40.5%, 44.6% and 49.2% respectively. In SA, glasshouse experiments showed that pathotypes P1 and P4 could reduce seed yield by 35% and 82% respectively. Surveys of pulse crops over a number of years in Vic and SA showed that up to 15% of crops were infected with PSBMV, with a within crop incidence of up to 5% of plants. In surveys in WA in 1999, PSBMV was found in 42% of field pea crops and the level of virus detected in pea seed stocks was up to 63%. The surveys in WA indicated that PSBMV has a severe effect on seed quality of pulse crops with seed coat symptoms evident on >80% of faba bean, >50% of lathyrus species and field peas and 5% of chickpea seed. At NSW-DPI Tamworth, screening of pea germplasm in the field for resistance to PSBMV has shown that highly susceptible lines may be 100% infected late in the season (Van Leur, unpublished).
Symptoms
The disease is characterized by a mild mottling on the leaves, which is not easily recognizable because of the small size of the lentil leaf.
The affected plants are reduced in growth, which is easily recognized, especially when they are compared with healthy plants. Yield reductions of 15-16% could occur in some different cultivars. Seeds from infected plants occasionally show dark staining on the seed.
Lentils may show no symptoms or there may be chlorosis in new shoots, mottling on leaves, shoot tip necrosis and stunting of plants.
Hosts
The natural host range of PSBMV is limited to the Fabaceae. It infects temperate pulses (chickpea, faba bean, field pea, lentil) other legumes (garden pea, narbon bean) and pastures (lathyrus and vetch). A number of PSBMV pathotypes have been recognised by their ability to infect a number of pea differential genotypes.
Geographic distribution
Worldwide
Biology and transmission
The virus is believed to have spread worldwide through the exchange of infected seed. Seed transmission rates of up to 100% in peas and up to 44% in lentils have been reported. In Victoria, we have detected PSBMV at low levels in some commercial chickpea seedlots (0.4% of seed) and at higher levels in field pea and lentil seedlots (greater than 2% of seed). In the USA, 3% PSBMV infection in pea seedlots and 32-40% in lentil seedlots have been reported. At ICARDA, PSBMV was found to be transmitted through lentil seeds at rates of up to 44%. PSBMV is also transmitted in a non-persistent manner by more than 20 aphid species and by mechanical means. The most efficient vector is the pea aphid (Acyrthosiphon pisum). The other species are as follows: A. pelargoni, A. sesbaniae, Aphis craccivora, A. fabae, A. gossypii, A. nasturtii, Aulacorthum circumflexum, A. solani, Brevicoryne brassicae, Cryptomyzus ribis, Dactynotus escalantii, Macrosiphum avenae, M. euphorbiae, M. pisi, M. rosae, Metopolophium dirhodum, Myzus persicae, Ovatus crataegarius, Phorodon cannabis, Rhopalosiphum padi and Semiaphis dauci. In Victorian pulse crop surveys, we have found cowpea aphid (Aphis craccivora), foxglove aphid (Aulacorthum solani), and green peach aphid (Myzus persicae), which are all vectors of PSBMV.
Detection/indexing method in place at the CGIAR Center at ICARDA
- (TBIA) Tissue Blot ImmunoAssay test, Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be the main source of PSBMV, therefore sowing virus tested seed is the most effective way of controlling this virus. Commercial seed tests are available. Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses, such as PSBMV. Pulse crops should be sown away from other legumes to minimise the spread of PSBMV.
Procedure followed at the centers in case of positive test
- Destroying seed, producing healthy seed under controlled conditions
References and further reading
http://www.icarda.org/Publications/Field_Guides/Lentil/Lent7.Html
Scientific Name
Broad bean Mottle Virus (BBMV)
Significance
The virus was seen as being of minor importance until reports of its widespread occurrence began to appear in the 1970s and 1980s.
Symptoms
Three quarters of the plants displayed leaf symptoms, many were dwarfed and produced few or no flowers. Infected plants were in concentric patches, BBMV can affect seed quality by causing necrosis and shrivelling of the seed. Seed transmission rates in legumes are low and range from 0.1% to 1.4%.
Hosts
Under experimental conditions susceptibility to infection by virus is found in several families. Susceptible host species are found in the Family Amaranthaceae, Chenopodiaceae, Leguminosae-Papilionoideae, Solanaceae. The following species were susceptible to experimental virus infection: Chenopodium album, Chenopodium amaranticolor, Chenopodium hybridum, Chenopodium quinoa, Cyamopsis tetragonoloba, Glycine max, Gomphrena globosa, Lathyrus odoratus, Lens culinaris, Lupinus albus, Medicago sativa, Melilotus albus, Nicotiana clevelandii, Phaseolus vulgaris, Pisum sativum, Trifolium hybridum, Trifolium incarnatum, Trifolium pratense, Trifolium repens, Trifolium subterraneum, Vicia faba, Vicia villosa.
Geographic distribution
BBMV was reported in faba bean crops in Portugal, Sudan, Morocco, and Algeria. then undertook a regional survey and found BBMV in faba bean crops in Egypt, Morocco, Sudan, Syria and Tunisia. Fortass and Bos (1991) surveyed faba bean crops in Morocco and found that luteoviruses and BBMV were the most prevalent viruses.
Biology and transmission
BBMV survives between growing seasons of the primary pulse host in an alternative host or in infected seed. Due to the fact that the natural host range includes at least four families and includes many food legumes and non-legume wild hosts, it may survive in a range of alternative hosts.
Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by grafting.
Virus is transmitted by arthropods, by insects of the order Coleoptera; Acalymma trivittata, Colaspis flavida, Diabrotica undecimpunctata, Sitona lineata.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Tissue blot immunoassay (TBIA)
Treatment/control
- Seed is considered to be the main source of BBMV, therefore sowing virus tested seed is the most effective way of controlling this virus. Commercial seed tests are available. Chemical control of insects is not an effective method for controlling virus.
Procedure followed at the centers in case of positive test
- Destroying seed, producing healthy seed under controlled conditions
References and further reading
http://www.padil.gov.au/pbt/index.php?q=node/46&pbtID=152
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.010.0.03.002.htm
Comments
- No comments found





Leave your comments
Post comment as a guest