Insects - groundnut
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Peanut bruchid beetle, Groundnut bruchid, Tamarind weevil, Groundnut borer, Seed beetle
Scientific name
Caryedon serratus Olivier.
Other scientific names
Caryedon gonagra, Caryedon fuscus, Caryedon languidus, Pachymerus sibutensis, Careydon sibutensis, Caryedon acaciae, Pachymerus conger, Bruchus serratus, Caryedon gonager, Caryoborus gonagra, Pachymerus gonagra, Caryoborus gonager, Caryoborus serratus, Pachymerus serratus.
Importance
High
Significance
Caryedon serratus is a serious pest of stored groundnuts, particularly when these are still in their shells. The damage caused is particularly significant when the nuts are destined for confectionery purposes.
Symptoms
The translucent milky-white eggs are attached to the pod wall. After hatching, the larva burrows straight through the egg shell and the pod wall, and starts eating the seed. The first sign of attack is the appearance of ‘windows’ cut into the pod wall by the larva to allow the adult to leave the pod after emerging from the pupal cacoon. Fully grown larva sometimes come out through the exit holes made by the previous generations. They often live in the storage sacks and pupate in large numbers at the bottom of the pile of sacks. By this stage, the groundnut seeds are severely damaged for human consumption or oil expulsion (Wightman and Ranga Rao 1993).
Host
Arachis hypogaea (groundnut), stored products (dried stored products), Elaeis guineensis (African oil palm), Gossypium (cotton), Phaseolus (beans), Theobroma cacao (cocoa) and Tamarindus indica (Indian tamarind).
Geographical distribution
Caryedon serratus is of Asian origin, but is distributed to many tropical and subtropical regions of the world (Southgate 1979). Although it is especially prevalent in the warm and hot parts of Asia, North-eastern and West Africa, the West Indies, Hawaii, and parts of South and Central America as far north as Mexico, it is a serious pest of stored groundnuts only in West Africa.
Biology and transmission
The eggs of C. serratus are translucent, white, oval, approximately 1 mm long and 0.5 mm wide (Davey, 1958). The larvae are scaribeiform and sparsely hairy. They usually leave the pods of their host before pupation. Pupae are creamy white, glabrous, about 5 mm long (Cox, 1996). It is a large robust bruchid which, in commerce, is almost always associated with groundnuts or tamarinds. It has a reddish-brown cuticle, densely clothed with grey-brown setae, but with dark, irregular markings on the elytra. The pygidium in the female is fully visible from above. Body length is 3.5-7.4 mm. Almost entirely covered dorsally by golden scale-like setae. Antennae are 5 to 10 serrate with 2-4 segments impressed basally. Head is with prominent and median carina. Pronotum is subconical, evenly convex dorsally, reddish-fuscous to testaceous, irregularly punctured, with fine bead around all margins, except for anterior angles. Elytra is one-and-a-half times as long as broad, puctate-striate, testaceous to dark reddish-fuscous usually with darker maculatron. Metafemora strongly thickened, with ventral, comb-like row of one large, sub median tooth followed by 8-12 small teeth. Metatibiae strongly curved, but simple, without either ventral, sub basal tubercle or two small, unequal, ventroapical calceriae (Prevett 1967). The optimum conditions for development are 30-33°C and 70-90% RH, under which conditions the development period is 41-42 days. Breeding can take place between 23 and 35°C (Davey 1958).
Detection/indexing methods at ICRISAT
- Dry seed examination using magnifying lens and X-ray radiography are used.
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed Seed treatment with chlorpyriphos 3-g/kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Removal of the infested seeds followed by the seed treatment.
References and further reading
Cox ML. 1996. The pupae of Chrysomeloidea. In Jolivet PHA, Cox ML, eds., Chrysomelidae Biology, vol. 1: The Classification, Phylogeny and Genetics, pp 119-265. Amsterdam, The Netherlands: SPB Academic Publishing.
Davey PM. 1958. The groundnut bruchid, Caryedon gonagra (F.). Bulletin of Entomological Research, 49(2):385-404.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Prevett P. 1967. The larva of Caryedon serratus (Ol.), the groundnut seed beetle (Coleoptera: Bruchidae). Journal of Stored Products Research 3:117-127.
Southgate BJ. 1979. Biology of the Bruchidae. Annual Review of Entomology 24:449-473.
Whiteman JA, Ranga Rao GV. 1993. A groundnut insect identification handbook for India. Information Bulletin no. 39. Patancheru, AP 502324, India: International Crops Research Institute for the Semi-Arid Tropics. 64pp.
 Peanut Bruchid Beetle (Caryedon serratus) of groundnut: (A)milky-white eggs on the pod; (B)windows cut into the pod wall by the larvae and (C)adult (photos:ICRISAT) |
Red Flour Beetle, Rust-red flour beetle
Scientific name
Tribolium castaneum Herbst.
Importance
Low
Significance
Red flour beetles attack stored grain products such as flour, cereals, meal, crackers, beans, spices, pasta, cake mix, dried pet food, dried flowers, chocolate, nuts, seeds, and even dried museum specimens. These beetles have chewing mouthparts, but do not bite or sting. The red flour beetle may elicit an allergic response, but is not known to spread disease and does not feed on or damage the structure of a home or furniture. These beetles are the most important pests of stored products in the home and grocery stores.
Symptoms
Infestation by adult beetles can be readily observed by the tunnels they leave when they move through the flour and other granular food products. When infestation is severe, these products turn grayish-yellow and become moldy, with a pungent odor. Infestation may also be apparent by the appearance of adults on the surface of the seeds (Whiteman and Ranga Rao, 1993).
Hosts
Arachis hypogaea (groundnut), Avena sativa (oats), Bertholletia excelsa (Brazil nut), Hordeum vulgare (barley), Juglans (walnuts), Lens culinaris (lentil). Oryza sativa (rice), Phaseolus (beans), Phaseolus lunatus (lima bean), Pisum sativum (pea), Prunus dulcis (almond), Secale cereale (rye), Triticum (wheat), Triticum spelta (spelt), Zea mays (maize).
Geographic distribution
The rust-red flour beetle, originally of Indo-Australian origin, has a cosmopolitan distribution but occurs more in warmer climates. Its distribution is mainly in Africa, Australasian – Oceanian, Central and South America, Europe, Northern Asia, Mediterranean Basin, South and South-east Asia, USA and Canada. It is found in stored grain, seeds, flour, dried fruits, nuts (Teetes et al. 1983).
Biology and transmission
The adult is 3-4 mm long, oblong, and brown in color. It lays about 450 eggs, distributed among the pods and seeds. The eggs are minute, cylindrical and white. The eggs hatch in 3-4 days, and the slender cylindrical larvae start feeding on the seed. Pupation takes place in the produce without a cacoon. The pupal period may last for 7-10 days, and the adult can live up to 18 months. The developmental period from egg to adult may require about 20 days at 30°C with about 70%RH (Whiteman and Ranga Rao 1993).
Detection/indexing methods at ICRISAT
- X-ray radiography is used for suspected samples because it offers a non-destructive method. Dry seed examination using magnifying lens to separate the infested seed.
Treatment/control
- Fumigation of the samples with methyl bromide by 32 g/m3 for 4 hr followed by treatment with chlorpyriphos at 3-g/kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Infested samples are rejected.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P., India: International Crops Research Institute for Semi-Arid Tropics. 125pp.
Whiteman JA, Ranga Rao GV. 1993. A groundnut insect identification handbook for India. Information Bulletin no. 39. Patancheru, AP 502324, India: International Crops Research Institute for the Semi-Arid Tropics. 64pp.
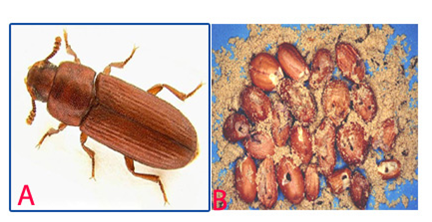 Red Flour Beetle (Tribolium castaneum) of groundnut: (A)adult and (B)damaged seed (photos:ICRISAT) |
Scientific name
Trogoderma granarium Everts.
Other scientific names
Trogoderma afrum, Trogoderma khapra, Trogoderma quinquefasciata
Importance to CGIAR Centers
High
Significance
Trogoderma granarium is a serious pest of stored products under hot dry conditions. Established infestations are difficult to control because of the beetle's ability to live without food for long periods of time and to survive on foods of low moisture content, its habit of crawling into tiny cracks and crevices and remaining there for long periods, and its relative tolerance to many surface insecticides and fumigants.
Symptoms
The khapra beetle is one of the world's most feared stored-product pests. The obvious signs of a khapra beetle infestation are the larvae and cast skins. Larvae and adults are best identified by microscopic examination. Larvae are mostly seen just before dusk, since they are more active at that time (Anonymous 1981).
Host
Larvae feed on a wide variety of stored products and dried foods. They prefer whole grain and cereal products such as Triticum aestivum (wheat), Hordeum vulgare (barley), and Oryza sativa(rice), but larvae have been recorded on the following: Avena spp. (oats), Secale spp. (rye), Zea mays (corn), dried blood, dried milk, fishmeal, Arachis hypogea (groundnut), flour, bran, malt, Linum usitatissimum (flax seed), Medicago sativa (alfalfa seed), Lycopersicum esculantus (tomato seed), Phaseolus vulgaris (pinto beans), Vigna unguiculata (blackeyed cowpeas), Sorghum bicolor (sorghum seed) and many other food products (Lindgren and Vincent 1959; Lindgren et al. 1955).
Geographic distribution
The distribution of khapra beetle extends from Mayanmar (Burma) to West Africa and is limited by the 35° parallel to the north and the equator to the south. It has been introduced by commerce into some areas of similar climatic conditions (Anonymous 1981). The khapra beetle is found in all continents where grain and grain products are stored.
Biology and transmission
The adults are oblong-oval beetles, approximately 1.6 to 3.0 mm long and 0.9 to 1.7 mm wide. Males are brown to black with indistinct reddish brown markings on elytra. Females are slightly larger than males and lighter in color. The head is small and deflexed with a short 11-segmented antenna. The antennae have a club of three to five segments, which fit into a groove in the side of the pronotum. The adults are covered with hairs. The eggs are milky white, turning pale yellowish with age, cylindrical, 0.7 ´ 0.25 mm, one end rounded, the other pointed and bearing spine-like projections. Larvae are uniformly yellowish white, except head and body hairs are brown. As the larvae increase in size, their body color changes to a golden or reddish brown, more body hairs develop, and the tail becomes proportionally shorter. Mature larvae are approximately 6 mm long and 1.5 mm wide. Adult khapra beetles have wings, but apparently do not fly and feed very little. Mated females live from four to seven days, unmated females from 20 to 30 days, and males from seven to12 days. Mating occurs about five days after emergence, and egg laying begins almost immediately at 40°C. Egg laying may begin at one to three days at cooler temperatures, but no eggs are produced at 20°C. Eggs hatch in three to 14 days after the female lays an average of 50 to 90 eggs that are loosely scattered in the host material. Complete development from egg to adult can occur from 26 to 220 days, depending upon temperature. Optimum temperature for development is 35°C. If the temperature falls below 25°C for a period of time or if larvae are very crowded, they may enter diapauses. They can survive temperatures below -8°C. In diapauses, the larvae can molt but are inactive and may remain in this condition for many years (Anonymous 1981).
Detection/indexing methods at ICRISAT
- Dry seed examination using magnifying lens
Treatment/control
- High concentrations of fumigant (Aluminium phosphide) are maintained during the fumigation period to allow penetration into all cracks and crevices. In an eradication program, both fumigants and surface treatment (chloropyriphos) are used in combination with preventive measures, e.g., good sanitation practices and exclusion.
Procedures followed in case of positive test at ICRISAT
- Rejection and incineration of the infested seed samples.
References and further reading
Anonymous. 1981. Data sheets on quarantine organisms. Trogoderma granarium Everts. European and Mediterranean Plant Protection Organization Bulletin 11 (1) Set 4, List A2, 1-6 pp.
Lindgren DL, Vincent LE. 1959. Biology and control of Trogoderma granarium Everts. Journal of Economic Entomology 52: 312-319.
Lindgren DL, Vincent LE, Krohne HE. 1955. The khapra beetle, Trogoderma granarium Everts. Hilgardia 24: 1-36.
 Khapra Beetle (Trogoderma granarium) of groundnut: (A)adults and (B)larvae on seeds (photos: www.agspsrv34.agric.wa.gov.au) |
Comments
- No comments found





Leave your comments
Post comment as a guest