main
Characterization standards
Contact person for characterization standards: Hari D Upadhyaya, ICRISAT, India
Contributors to this page: ICRISAT, Patancheru, India (Hari D Upadhyaya, Shivali Sharma); Bioversity International, Montpellier (Elizabeth Arnaud); Bioversity International, Rome (Adriana Alercia); CIMMYT, Mexico (Suketoshi Taba); CIP, Peru (David Tay); ICARDA, Syria (Kenneth Street); IRRI, Los Baños, Philippines (Ruaraidh Sackville Hamilton) and crop experts.
External reviewer: Murari Singh.
Introduction
Plant genetic resources cover landraces, obsolete varieties and wild species, and provide basic materials to the crop experts to use genetic variability for the development of high yielding cultivars with a broad genetic base. However, the utilization of these genetic resources depends upon their efficient and adequate characterization and evaluation, which requires efficient characterization standards and appropriate strategies.
Characterization and evaluation document the diversity in descriptive traits which vary with the species. In order to facilitate standardization of information obtained during characterization, Bioversity International has been coordinating the development, publication and update of various plant descriptor lists in close cooperation with crop experts and genebank curators (see also the crop descriptors on the Bioversity website by clicking here). There are descriptor lists developed for more than 90 crops. Guidelines are also available for developing crop descriptor lists. Characterization is also increasingly done using complementary characterization methods to capture the full information on a broad range of traits.
Phenotypic characterization strategies and standards should be reviewed at regular intervals to determine their value and usefulness in characterization. This is best done as a collaborative process with collaborators and crop experts:
- Establish a task force of crop experts for the crop.
- Share existing descriptor lists and guidelines for developing descriptors with the task force.
- Obtain opinions and feedback from the task force members and collaborators on the descriptors and their usefulness and propose changes.
- Modify the descriptors' state and stage of recording data, based upon the responses.
Based on this process, modified descriptor lists were developed by crop experts and are available for chickpea, rice, maize, potato, Musa, pigeonpea, sorghum and sweet sorghum and are open for further discussion.
Analysis of diversity data
Analysis of trait data generated from characterization and evaluation studies is used to understand and use diversity. A large number of distance measures are available for analyzing similarity/dissimilarity among accessions based on different traits representing different types of variables, and the selection of the most appropriate distance measure for each trait is the prerequisite for diversity analysis studies. One of the approaches is to form clusters where accessions between clusters would be more diverse than the accessions within a cluster. The clustering algorithms require a distance/similarity matrix between the accessions which can be calculated depending upon the nature or type of traits such as morphological and agronomic traits and/or molecular markers. For more information click here.
Types of data
A. Morphological traits: Data recorded on morphological traits, such as flower colour, pigmentation, seed colour etc. represent discrete or categorical variables and can be grouped as:
a. Binary: presence or absence of a characteristic.
b. Nominal: colour or shape of a trait.
c. Ordinal: a visual scale arranged to represent the intensity of a trait.
B. Agronomic traits: Data recorded on agronomic traits such as plant height, 100-grain weight, yield per plant, etc. represent continuous variables.
C. Molecular marker: The data on molecular markers is recorded in the following two forms:
a. Binary data: presence or absence of molecular marker bands.
b. Allelic data (i.e. on allele size).
Strategy for data analysis
-
Each distance measure has its own properties and assumptions.
-
The genetical context and mathematical properties of similarity/dissimilarity measures should be given importance when choosing a measure.
-
Different distance measures provided different estimates of mean, minimum and maximum diversity.
-
Ward’s method: Useful in clustering accessions for morphological and agronomic traits.
-
Different distance measures resulted in different number of clusters for different traits, however, a relatively higher number of accessions tend to cluster together even when different matrices/methods were used.
A Helpdesk is available at http://220.227.242.211:9905/ to facilitate system-wide common procedures for diversity analysis across genebanks. This Helpdesk also serves the genebank community globally by providing basic information on various aspects of diversity analysis, such as selection of appropriate similarity/dissimilarity matrices, cluster analysis, analyzing diversity using individual trait and/or combination of traits.
Suggested analyses for trait types
|
Traits |
Distance measure |
Remarks |
|
Morphological traits |
Simple matching |
Only studied distance measure for nominal traits. |
|
Agronomic traits |
Euclidean |
Identified the same pair of accessions exhibiting minimum diversity but different pair of genotypes exhibiting maximum diversity. |
|
|
||
|
D2 Mahalanobis |
Takes into account the correlations of the datasets and is scale-invariant i.e. not dependent on the scale of measurement. |
|
|
Molecular markers |
|
|
|
Allelic data |
Simple matching |
The mean, as well as range, of diversity was reduced, so could not discriminate the pair of accessions exhibiting maximum diversity. |
|
Euclidean |
Identified the same pair of accessions exhibiting minimum and maximum diversity. |
|
|
Roger’s |
||
|
Chord 67 |
Assumption: mutation rate is small and variation in selection pressure is rapid and haphazard i.e. no constant direction in allele frequency changes, which is not fulfilled in seed banks and plant breeding material that have evolved due to directed selection pressure rather than rapid and haphazard changes. |
|
|
Chord 69 |
|
|
|
Binary data |
Dice |
Identified the same pair of accessions having minimum and maximum diversity. |
|
|
Jaccard |
Jaccard is the most appropriate when the purpose of measure of similarity/dissimilarity is to indicate how similar/different the objects are with respect to attributes present (coded as 1) and to ignore the impact of attributes absent (0). |
|
|
Simple matching |
Based on the assumption that all shared bands (both presence and absences) are taken into account irrespective of the reason why bands are absent. |
|
Combination of traits |
|
|
|
Morphological (nominal) + Agronomic traits (continuous) |
Gower’s distance |
Simultaneous use of variables of different scales of measurement (nominal, continuous, binary) in the estimation of similarity/dissimilarity has the ability to accommodate mixed data types and, due to its metric qualities and flexibility, it can be modified to include negative matches in the estimation of similarity by simply modifying the binary weighting system. |
|
Morphological (nominal) + molecular data (binary) |
Gower’s distance |
|
|
Agronomic (continuous) + molecular (binary) |
Gower’s distance |
|
|
Morphological (nominal) + agronomic (continuous) + molecular data (binary) |
Gower’s distance |
|
|
Cluster analysis |
|
|
|
Ward’s minimum variance method |
|
Found more useful in chickpea as it grouped the genotypes into defined clusters. |
|
UPGMA (Unweighted Pair Group Method using Arithmetic averages) |
|
In chickpea, genotypes were not grouped into clusters. |
References and further reading
Cavalli-Sforza LL, Edwards AWF. 1967. Phylogenetic analysis: Models and estimation procedures. American Journal of Human Genetics 19:233–257.
Dice LR. 1945. Measures of the amount of ecologic association between species. Ecology 26:297–302.
Gower JC. 1971. A general coefficient of similarity and some of its properties. Biometrics 27:857-874.
Hair JR, Anderson RE, Tatham RL, Black WC. 1995. Multivariate data analysis with readings. 4th Edition, Prentice Hall, Englewood Cliffs, NJ.
Jaccard P. 1908. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 44:223–270.
Malecot G. 1948. Les Mathématiques de l'Hérédite. Masson et Cie, Paris.
Mohammadi SA, Prasanna BM. 2003. Analysis of genetic diversity in crop plants — Salient statistical tools and considerations. Crop Science 43:1235–1248.
Payne RW. 2009.The Guide to GenStat® Release 12, Part 2: Statistics. VSN International, 5 The Waterhouse, Waterhouse Street, Hemel Hempstead, Hertfordshire HP1 1ES, UK.
Reif JC, Melchinger AE, Frisch M. 2005. Genetical and mathematical properties of similarity and dissimilarity coefficients applied in plant breeding and seed bank management. Crop Science 45:1-7.
Rogers JS. 1972. Measures of genetic similarity and genetic distance. p.145–153. In Studies in genetics VII. Publ. 7213. Univ. of Texas, Austin, USA.
Sneath PHA, Sokal RR. 1973. Numerical taxonomy. Freeman, San Francisco, CA, USA.
Ward JH. Jr. 1963. Hierarchical grouping to optimize an objective function. J. Am. Statist. Assoc. 58:236-244.
Upadhyaya HD, Sarma NDRK, Ravishankar CR, Albrecht T, Narasimhudu Y, Singh SK, Varshney SK, Reddy VG, Singh S, Dwivedi SL, Wanyera N, Oduori COA, Mgonja MA, Kisandu DB, Parzies HK, Gowda CLL. 2010. Developing mini core collection in finger millet using multilocation data. Crop Science (Accepted).
Transgene analysis
Contributors to this page: CONABIO, Mexico (Francisca Gasman); IITA, Nigeria (Maria Ayodele); CIMMYT, Mexico (Etienne Duveiller, Huixia Wu, Jonathan Crouch, Monica Mezzalama, Suketoshi Taba, Thomas Payne); Bioversity International, Italy (Ehsan Dulloo); International Seed Federation Representative, Monsanto, Mexico (Juan de la Fuente); CIP, Peru (Marc Ghislain); CIMMYT, Kenya (Stephen Mugo); IRRI, Philippines (Ruaraidh Sackville Hamilton); Danish Seed Health Centre for Developing Countries, Denmark (Jan Torp).
This page shows the latest updates on the development of crop-specific guidelines to maintain the genetic identity of germplasm as received and collected regarding the possible unintentional presence of transgenes.
Here we use the term transgenic organism as synonymous to a living modified organism following the Cartagena Protocol on Biosafety. Transgenic varieties have increasingly taken part of modern agriculture, also in developing countries. Several CGIAR Centres currently have genetically engineered material under development to make available the benefits of modern biotechnology to resource-poor farmers. However, voices of concern have urged us to guarantee the maintenance of the genetic identity of the germplasm collected, received and conserved with particular emphasis on avoidance of the unintentional presence of transgenes.
In this context, it is very important for genebanks to prevent the unintentional introgression of exotic genes, including transgenes, not already present in samples conserved in their collections. Best practices in genebanks should be able to achieve a high degree of probability that an accession maintains its genetic identity over generations.
This activity, aimed at limiting the unintentional presence of exotic genes including transgenes into genebank collections, is in line with agreed CGIAR guiding principles. It includes the creation of a database of field-tested genetically modified (GM) crops worldwide, environmental release status and methods of detection and development of crop-specific approaches to maintain germplasm free from transgenes.
|
Workshop report |
|
Click to open PDF (0.1 MB) |
The information on this page was coordinated by Monica Mezzalama (CIMMYT) and implemented by CGIAR Centres holding crops of relevance [Ruaraidh Hamilton (IRRI, Philippines), Marc Ghislain (CIP, Peru) and Suketoshi Taba (CIMMYT-maize collection)] with the additional participation of external experts (see the full list of contributors at the top of this page). This was implemented in line with the recommendations of the CGIAR Genetic Resources Policy Committee.
A workshop held in Mexico (15-17 August 2007) gathered relevant experts who, on the basis of the “Guiding principles for the development of CGIAR Centres' policies to address the possibility of unintentional presence of transgenes in ex situ collections”, drafted the document “Develop crop-specific guidelines to maintain germplasm genetic identity”.
Guidelines to maintain germplasm genetic identity
The basic operations occurring in a genebank, from the acquisition of a new accession to final storage and distribution, are defined in the figure below. It is understood that for specific crops these operations may differ slightly.
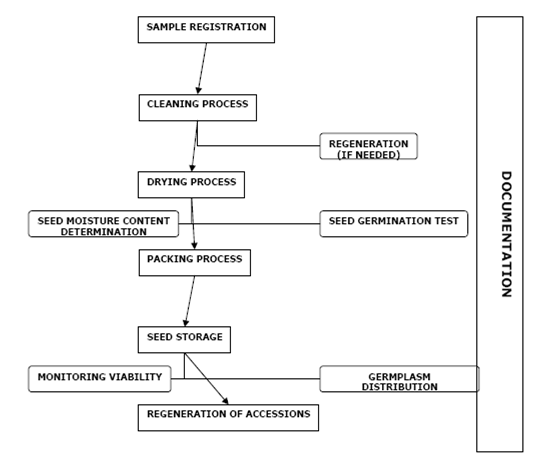
Sequence of procedures in a genebank
Possible risks
A risk regarding transgenes is the occurrence of an unintentional introduction of a transgene or transgenes into a genebank collection during each operation. Indoor/internal (contamination, mixture) and outdoor/external (other released germplasm, experimental germplasm) sources of this risk associated with each operation have been identified and described.
A general schematic description of the guidelines' risk management measures at a non-crop-specific level is shown here.
The establishment of crop-specific regulations was developed in accordance with national and institutional biosafety laws and regulations for maize (prepared by S. Taba), rice (prepared by R. Sackville-Hamilton) and potato (prepared by M. Ghislain) and reviewed and finalized by M. Mezzalama.
Testing procedures
Laboratory testing for GMO detection is recommended as a management measure, depending on the bank operation involved and on the identification and characterization of the source(s) of risk.
Different levels of testing procedures are suggested according to three levels of risk:
- High:
- Refusing introduction of an accession.
- Testing all germplasm to achieve the highest level of confidence.
- Testing using small initial sample size.
- Medium: testing using partial or probabilistic sampling methods to achieve a situation-specific result (depending on crop, source, etc.) at a defined level of confidence.
- Low: no testing, because either no transgenic events are present in the area where the sample was grown or there is no source of risk of gene flow.
Other risk management measures that do not involve testing are described for each operation.
Testing to detect unintentional transgenes
Sample size
The decision on the sample size to be tested should be guided by:
- Statistical indications that can be found in Hernandez-Suarez et al. (2008) and other sources (e.g.,GIPSA, ISTA; see website references).
- The amount of seed available.
- The testing procedures used.
How to test
The decision regarding how to test the material is crop-specific, but it is agreed that DNA-based (rather than protein-based) detection will be essential. It is predicted that in the future, all approved transgenes will have publicly and commercially available markers and detection procedures. Therefore, it is necessary to retrieve and maintain updated information on transgenic constructs and respective detection methods. A website with links to the most accredited external database(s) was prepared. Nevertheless, some of this construct information may be kept confidential and hence may not be public.
When to test
Testing should potentially be carried out at two key stages of genebank operations:
- On new acquisitions, including in situ collections and material originating ex situ, at the point of entry into a genebank.
- On existing accessions before initial use, regeneration and distribution.
Positive results and their publication
A cautious approach should be adopted, respecting the moral integrity of the partners in providing genetic material in good faith, while also recommending full transparency where the provider is aware of the presence of transgenes in the material provided.
Where a positive result is obtained, confidentiality and consultation with the provider in the first instance must be respected in order to obtain all necessary information regarding the material.
Genebank managers should follow the same principles as for the unintentional presence of pathogens and not become a “global GM detection police”.
- Material found positive should usually be destroyed and the provider informed.
- If the material is accepted by the genebank, the center also has a moral obligation to inform the national biosafety authority.
If a sample tests positive: Crisis management responses
- For a new acquisition the following possibilities are suggested:
- Retest to verify.
- Destroy or return to the provider.
- Maintain the sample noting the possible presence of the transgene(s).
- Scrutinize possible sources of contamination.
- Enter into a confidential dialogue with the provider (or donating country authority).
- If the material is accepted into the genebank, national and institutional regulations must be followed, with public (transparent) release of all information.
- For an existing accession the following possibilities are suggested:
- Retest to verify.
- Identify the gene constructs.
- Determine level of presence of the transgenes.
- Inform past recipients, if the accession has previously been distributed.
- Inform the provider (without accusing).
- Inform other genebank holders of the accession (identified through crop registries).
- Maintenance of the sample will depend on the individual bank: as is, or “cleaned” if deemed necessary. Follow guidelines for recognized transgenic accessions.
- Try to minimize any institutional damage.
- For an existing accession that has been distributed and is in a commercialized form, the following suggestions are made:
- Formation of a crisis-management team [(including institutional management, legal advisor, communications expert, biosafety expert, scientist(s)].
- Use of a crisis management reaction matrix (a tool for PR management) to produce a communication strategy.
- Prompt (proactive) timing is essential.
- Issue a global public alert.
- High transparency will result in fewer negative consequences than secrecy. Transparency should also be maintained during ordinary times by cultivating public media that are informed on the types and aims of GM research at CGIAR Centres, as an “educational” activity that may help ameliorate the impact when an undesirable result must be made public.
GMO-free declaration
GMO-“free” statements cannot be issued for technical reasons, except for clonally propagated crops (at high costs). However, transparency is essential and may require a clear statement of procedures used to ensure that material has a low probability of containing transgenes.
Declarations can be issued within stated thresholds and must include stipulations such as “to the best of our knowledge”.
References and further reading
Hernandez-Suarez CM, Montesinos-Lopez O, Crossa J, McLaren G. 2008. Probability models for detecting transgenic plants. Seed Science Research 18:77–89.
Guiding principles for CGIAR Centres' policies to address the possibility of unintentional presence of transgenes in ex situ collections. Available from: http://www.sgrp.cgiar.org/sites/default/files/CGRFA11_Guiding-principles[1].pdf. Date accessed: 22 March 2010.
Booklet of CGIAR Centre Policy Instruments, Guidelines and Statements on Genetic Resources, Biotechnology and Intellectual Property Rights. Statements on Genetic Resources, Biotechnology and Intellectual Property Rights, Version II - Rome, July 2003, 51 pp. Available here.
Interesting websites
The following websites provide descriptions of transgenic events and databases on the events released for each crop in each country; the international biosafety agreement; and technical information on sampling and testing.
Convention on Biological Diversity
Cartagena Protocol on Biosafety
Grain Inspection, Packers and Stockyards Administration (GIPSA): Homepage - Sampling procedures
International Service for the Acquisition of Agri-biotech Applications (ISAAA)
International Seed Testing Association (ISTA)
Genetic identity
Maintenance of the genetic identity of accessions during genebank management is one of the cornerstones of genetic resources conservation. Accessions maintained in genebanks should remain as genetically similar to the original collected material as possible. It is very important to prevent the unintentional introgression of exotic genes, including transgenes, not already present in samples conserved in germplasm collections. Genebank processes are designed to avoid inadvertent introduction of genes through geneflow, avoid selection and retain genes in low frequencies within the population to ensure that maximum diversity is retained for future use. Best practices in genebanks should be able to achieve a high degree of probability that an accession maintains its original genetic identity over generations of regeneration and storage.
Genetic integrity can be at risk at all stages of the genebank handling process and careful handling procedures should be put in place to reduce the risk of loss of alleles or genes within the population or accession. These include paying attention to uneven loss of genotypes during storage by ensuring viability does not decline to low levels, using adequate sample size to capture genes in low frequencies for regeneration, providing isolation from pollen contamination and reducing the regeneration interval and carrying out regeneration in environments where the germplasm is well adapted to minimize selection and genetic drift during regeneration.
Even with careful handling, accessions may face genetic erosion or lose genetic integrity during long term storage over many cycles of regeneration. Routine testing schemes are required to monitor genetic identity and genetic erosion during genebank handling. In the past these tests have relied on checking morphological traits against standard lists of descriptors and comparing the traits in each generation to the original phenotype or population. New molecular tools have now provided the opportunity to monitor genetic integrity at the genotype level and laboratory tests are available to determine any unintentional genetic erosion or change in genetic identity.
This section provides information on the standards used to describe diversity and the methods used to detect changes in genotype, unintentional loss of alleles or genes or unintentional inclusion of genes and transgenes into accessions. Information is available on:
References and further reading
Hirano R, Jatoi SA, Kawase M, Kikuchi A, Watanabe N. 2009. Consequences of ex situ Conservation on the Genetic Integrity of Germplasm Held at Different Gene Banks: A Case Study of Bread Wheat Collected in Pakistan. Crop Science 49:2160-2166.
Genetic integrity
Contact person for Genetic integrity: Jessica Rey, IRRI, Philippines
Contributors to this page: IRRI, Los Baños, Philippines (Jessica Rey, Kenneth McNally, Ruaraidh Sackville Hamilton); Bioversity International, Montpellier (Elizabeth Arnaud); CIMMYT, Mexico (Suketoshi Taba); CIP, Peru (David Tay); ICARDA, Syria (Kenneth Street); ICRISAT, Patancheru, India (Hari D Upadhyaya).
Overview
The magnitude of genetic differences between supposedly duplicate samples is disturbingly high, even unacceptably high. All causes of genetic change are significant contributing factors – genetic drift, unintentional selection, pollen contamination, seed contamination, and mislabelling. In particular, analysis has demonstrated an unexpectedly high rate of mislabelling, a risk that existing “best practices” for genebank management have failed to manage. Similarly, existing best practices to not address the loss of diversity of genes for flowering date noted in the maize dataset.
The greatest changes in genetic composition are apparent between duplicates maintained at different genebanks; the differences are such that “duplicates” at different genebanks should perhaps be described as “equivalents” rather than “duplicates”. Significant changes also occur during management within a genebank.
Thus it is important and urgent to improve both the handling of accessions within genebanks and the transfer of accessions between genebanks, and to develop a strategy and protocols to do so.
General recommendations
- Germplasm handling standards need to be significantly raised in all genebanks through implementation of appropriate quality management systems (linked to activity 1.1 in the risk management section and 2.3.1 regeneration procedures section).
- “Rationalization” among seed genebanks sharing crops in common, in the sense of eliminating or combining duplicates that have the same historical origin, is strongly deprecated. Eliminating or combining duplicates may only be considered if supported by DNA data demonstrating biological duplication, not only historical duplication. However, the cost of genotyping to demonstrate biological duplication still remains very high compared to the cost of seed conservation. This would be particularly true, if a large number of markers are required to capture minor differences in two seemingly duplicate accessions (in several instances even a phenotypically dissimilar accession (through having the same number) may be difficult to differentiate using markers. Systematic identification and elimination of biological duplicates therefore remains an ineffective, inefficient strategy for species with conventional seed that can be conserved long-term in cold stores.
- Other forms of “Rationalization” among seed genebanks sharing crops in common may be considered, provided they are not affected by the presence of genetic differences between equivalent accessions. For example, responding to seed requests could be rationalized by redirecting them to the most convenient source for the recipient, provided there is no specific reason for preferring the specific samples of equivalent accessions held at other genebanks.
- Eliminating duplicates within and among genebanks holding collections of clonal crops is justified because of the high cost of conservation, but only when supported by rigorous molecular characterization.
- Serious consideration should be given to the development of low-cost “DNA barcoding” technologies and to their incorporation as routine checks during genebank management. For example, ideally:
- Every new seedlot produced after a cycle of regeneration should be screened and compared against its parent or most original sample, to assure maintenance of genetic integrity. This is important, although it will add to the cost.
- Every transfer of seed between genebanks should be accompanied by data specifying the DNA barcode of the original seed source, to be verified by the receiving genebank on incorporating the seed into its collection.
- In terms of throughput and cost-effectiveness, SNP chips are now considered the most suitable technology for tracking germplasm and quantifying their genetic integrity.
- Low density (96 SNP) chips are effective for detecting mislabelling errors.
- Higher density chips are needed for the other factors contributing to loss of genetic integrity.
- Serious consideration should also be given to other methods to promote accurate germplasm tracking during genebank operations, such as barcoding all genebank operations and/or the use of high-precision GPS to identify plots without relying on plot labels.
Recommendations for clonal crops
Model: Musa
For misclassification
- Using molecular markers combined with microscopic determination of ploidy level, check the classification of every newly received sample before introducing it into the collection.
- Verify before accessions are made available for distribution. DArT could be an appropriate tool, but the cost and the practicalities of DArT markers (in 96-well plates), for the moment, still do not make it appropriate for routine use.
- The new recommendation for Musa is now to test incoming material by SSR markers and ploidy determination.
For mislabelling
- Regularly analyze accessions using SSR or DArT.
- Request recipients of germplasm to provide effective feedback on the comparison of the received material in the field with photos/descriptions of the original sample.
- Every ten years, verify the morphology of the accessions maintained in vitro (medium-term conservation), by growing them on the field. This would thus represent 1/10th of the collection each year.
For off-types
- So far, molecular methods have not been effective for identification of off-types, or somaclonal variants.
- Morphological observations are required to detect these.
Thus during management of Musa, there is a need for more regular, specific morphological and molecular characterization to ensure adequate quality control.
Recommendations for seed crops
Existing FAO/IPGRI (1994) recommended standards for genebank management include these recommendations for seed increase:
- Regenerate only when required, either because of a loss of viability below a critical threshold (typically 85%) or because of a reduction in seed stocks below a critical threshold.
- Minimize the frequency of seed increase by ideally producing just enough seed to satisfy all seed requests before they begin to lose viability, so that regeneration will not be necessary until seed viability tests reveal a reduction in viability below 85%.
- Every cycle of seed increase almost inevitably results in some loss of genetic integrity, but we can and should avoid accumulating losses of genetic integrity over multiple generations of seed increase. As a general rule, avoid more than three successive generations.
These standards are supported, but should be more rigorously enforced and operationalized. It is recommended that the following elements of best practices be added:
- If seeds need to be multiplied early because seed stocks have been depleted sooner than 50% of the projected life span of seed in storage, double the plot size to store more seed from the next cycle.
- If seeds need to be multiplied because stocks have been depleted (not because of a loss of viability), then:
- Keep remnants of the parental seed for future seed increase – do not use them for distribution.
- For seed distribution, always use the youngest available seed lot of an accession.
- For seed multiplication, always use the oldest available viable seed lot of an accession.
- Conduct a final seed multiplication using the oldest seed lot at the moment its viability falls below 85%.
The maize data demonstrate a loss of diversity following regeneration, particularly in genes controlling flowering date; but they revealed no relation between the number of ears saved in the regenerated cycle and loss of genetic integrity among the accessions studied. This suggests that gene-specific selection is more important and random drift less important, (but it exists with inbreeding of the population, and should be preferably known with respect to the numbers of ears saved. However, it is usually assumed that outbreeding maize populations has a minimum level of inbreeding. Thus, a theoretical sampling strategy can still be employed to ensure conservation of the diversity present in the population. Inbred lines may need another study at SNP diversity level at various inbreeding cycles (as maize inbred can diverge in seed stocks maintained at the different seed genebanks and breeding stations) than has previously been supposed. It is concluded that the maize regeneration protocol should be modified to maintain the diversity of flowering dates unchanged.
In chickpea and rice, increases in average heterozygosity, as measured with Arlequin 3.0, were observed following regeneration. The results indicate that the consequences of cross-pollination and seed contamination are greater than have previously been supposed. It is common practice to ignore both factors, for example growing adjacent plots of self-pollinating crops with no control over cross-pollination. The results show this is inappropriate.
In rice it is known that almost all of the limited cross-pollination occurs over very short distances, between adjacent plots. Since 2007, the extent of cross-pollination between rice regeneration plots has been reduced in IRRI by sowing adjacent plots to varieties differing by more than two weeks in flowering date, so that a plot is never releasing pollen at the moment its neighbours have receptive stigmata: it has not been possible to evaluate the consequence of this management change during the current activity. Other methods need to be designed and introduced to reduce seed contamination.
Conclusion
A range of innovations is urgently needed to prevent the rapid losses of genetic integrity noted here. These include the introduction of new technologies such as barcoding, “DNA barcoding” with SNP chips and high-precision GPS. Morphological verification remains essential, both for somaclonal variants or other mutants that are difficult to identify with molecular methods, and for efficiently screening out obvious errors. In addition, we need a thorough re-evaluation of genebank management standards.
References and further reading
FAO/IPGRI. 1994. Genebank standards. Food and Agriculture Organization of the United Nations, Rome and International Plant Genetic Resources Institute, Rome. Available in English, Spanish, French and Arabic.
Hirano R, Jatoi SA, Kawase M, Kikuchi A, Watanabe N. 2009. Consequences of ex situ Conservation on the Genetic Integrity of Germplasm Held at Different Gene Banks: A Case Study of Bread Wheat Collected in Pakistan. Crop Science 49:2160-2166.
General genebank management strategies and principles
 |
Please click on one of the menu items on the left to proceed.
This section of the website covers important strategies and principles related to genebank decision-making, that are not necessarily crop-specific.
The pages listed on the left show the outputs developed by different groups, leaders of parallel activities of the project on the “Collective Action for the Rehabilitation of Global Public Goods in the CGIAR Genetic Resources System: Phase 2”, funded by the World Bank.
Some of these outputs are still under preparation, but will be posted as soon as they are released.
The persons responsible for the information provided in these pages are listed at the top of each page.





.jpg)