stog-pigeon-pea
Insects - pigeonpea
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Scientific name
Callosobruchus analis Fabricius.
Other scientific names
Bruchus analis, Bruchus glaber, Bruchus jekelii, Bruchus obliquus, Bruchus ciceri, Callosobruchus glaber, Callosobruchus jekelii.
Importance
Medium.
Significance
Infested chickpea lose their viability and are unfit for human consumption. In Africa, Asia, and Oceania, C. analis is considered a pest of economic importance for stored-legume grains (Southgate 1979).
Symptoms
Chickpea pods are seldom infested in the field. The pests attack nearly mature and dried pods during storage. The round exit hole and the white eggs on the pod wall are conspicuous. Infested stored seed can be recognized by the eggs on the seed surface, and the round exit holes with the ‘flap’ of seed coat.
Hosts
Cicer arietinum (chickpea), Cajanus cajan (pigeonpea) and other grain legumes.
Geographic distribution
Callosobruchus analis is wide spread in Asia.
Biology and transmission
Adults are small, 3 mm long brown beetles with black spots on the elytra. Eggs are laid on the seed surface. Larvae feed and pupate entirely within the seed. One generation is completed in 4-5 weeks (Ranga Rao and Shanower 1999).
Detection/indexing methods used in CGIAR
- Dry seed examination and X-ray radiography are used for detection of the bruchides.
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos 3 g kg-1 seed (Ghanekar et al. 1996).
Procedures followed at the centers in case of positive test
- Removal of the infested seeds followed by the seed treatment with chloropyriphos at 3 g kg-1 seed.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Ranga Rao GV, Shanower TG. 1999. Identification and Management of Pigeonpea and Chickpea Insects Pests in Asia. Information Bulletin No. 57, Patancheru, 502 324, A.P., India: International Crops Research Institute for the Semi Arid Tropics. 96pp.
Southgate BJ. 1979. Biology of the Bruchidae. Annual Review of Entomology 24: 449-473.
 Stored Grain Pest (Callosobruchus analis) of pigeonpea: (A)adult (photo: www.invasive.org/brows/detail=066007); (B)infested pods and (C)infested seed and white eggs (photos: ICRISAT). |
Scientific name
Trogoderma granarium Everts.
Other scientific names
Trogoderma afrum, Trogoderma khapra, Trogoderma quinquefasciata.
Importance
High.
Significance
Trogoderma granarium is a serious pest of stored products under hot dry conditions. Established infestations are difficult to control because of the beetle's ability to live without food for long periods of time and to survive on foods of low moisture content, its habit of crawling into tiny cracks and crevices and remaining there for long periods, and its relative tolerance to many surface insecticides and fumigants.
Symptoms
The khapra beetle is one of the world's most feared stored-product pests. The obvious signs of a khapra beetle infestation are the larvae and cast skins. Larvae and adults are best identified by microscopic examination. Larvae are mostly seen just before dusk, since they are more active at that time (Anonymous 1981).
 Khapra Beetle (Trogoderma granarium) of pigeonpea: (A)adults and (B)larvae and damaged grains (photos: www.agspsrv34.agric.wa.gov.au). |
Host
Larvae feed on a wide variety of stored products and dried foods. They prefer whole grain and cereal products such as Triticum aestivum (wheat), Hordeum vulgare (barley), and Oryza sativa(rice), but larvae have been recorded on the following: Avena spp. (oats), Secale spp. (rye), Zea mays (corn), dried blood, dried milk, fishmeal, Arachis hypogea (groundnut), flour, bran, malt, Linum usitatissimum (flax seed), Medicago sativa (alfalfa seed), Lycopersicum esculantus (tomato seed), Phaseolus vulgaris (pinto beans), Vigna unguiculata (blackeyed cowpeas), Sorghum bicolor (sorghum seed) and many other food products (Lindgren and Vincent 1959; Lindgren et al. 1955).
Geographic distribution
The distribution of khapra beetle extends from Mayanmar (Burma) to West Africa and is limited by the 35° parallel to the north and the equator to the south. It has been introduced by commerce into some areas of similar climatic conditions (Anonymous 1981). The khapra beetle is found in all continents where grain and grain products are stored.
Biology and transmission
The adults are oblong-oval beetles, approximately 1.6 to 3.0 mm long and 0.9 to 1.7 mm wide. Males are brown to black with indistinct reddish brown markings on elytra. Females are slightly larger than males and lighter in color. The head is small and deflexed with a short 11-segmented antenna. The antennae have a club of three to five segments, which fit into a groove in the side of the pronotum. The adults are covered with hairs. The eggs are milky white, turning pale yellowish with age, cylindrical, 0.7 ´ 0.25 mm, one end rounded, the other pointed and bearing spine-like projections. Larvae are uniformly yellowish white, except head and body hairs are brown. As the larvae increase in size, their body color changes to a golden or reddish brown, more body hairs develop, and the tail becomes proportionally shorter. Mature larvae are approximately 6 mm long and 1.5 mm wide. Adult khapra beetles have wings, but apparently do not fly and feed very little. Mated females live from four to seven days, unmated females from 20 to 30 days, and males from seven to12 days. Mating occurs about five days after emergence, and egg laying begins almost immediately at 40°C. Egg laying may begin at one to three days at cooler temperatures, but no eggs are produced at 20°C. Eggs hatch in three to 14 days after the female lays an average of 50 to 90 eggs that are loosely scattered in the host material. Complete development from egg to adult can occur from 26 to 220 days, depending upon temperature. Optimum temperature for development is 35°C. If the temperature falls below 25°C for a period of time or if larvae are very crowded, they may enter diapauses. They can survive temperatures below -8°C. In diapauses, the larvae can molt but are inactive and may remain in this condition for many years (Anonymous 1981).
Detection/indexing methods at ICRISAT
- Dry seed examination using magnifying lens
Treatment/control
- High concentrations of fumigant (Aluminium phosphide) are maintained during the fumigation period to allow penetration into all cracks and crevices. In an eradication program, both fumigants and surface treatment (chloropyriphos) are used in combination with preventive measures, e.g., good sanitation practices and exclusion.
Procedures followed in case of positive test at ICRISAT
- Rejection and incineration of the infested seed samples.
References and further reading
Anonymous. 1981. Data sheets on quarantine organisms. Trogoderma granarium Everts. European and Mediterranean Plant Protection Organization Bulletin 11 (1) Set 4, List A2, 1-6 pp.
Lindgren DL, Vincent LE. 1959. Biology and control of Trogoderma granarium Everts. Journal of Economic Entomology 52: 312-319.
Lindgren DL, Vincent LE and Krohne HE. 1955. The khapra beetle, Trogoderma granarium Everts. Hilgardia 24: 1-36.
Best practices for the safe transfer of pigeonpea germplasm
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Seed health testing protocol at ICRISAT-PQL for import
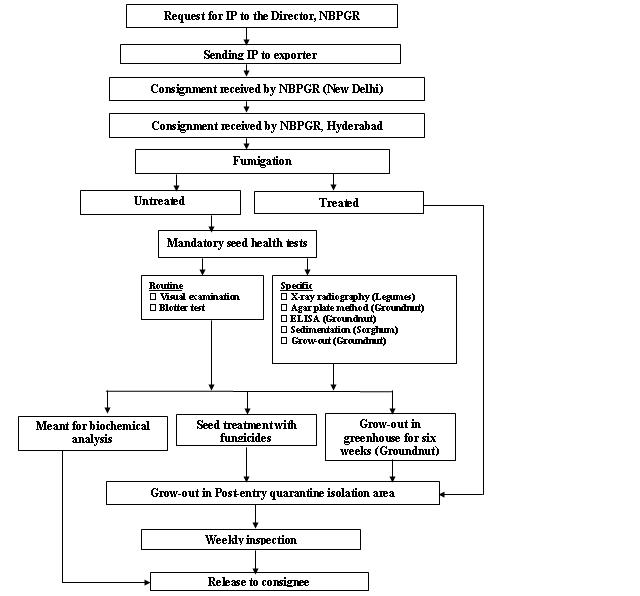
Seed health testing protocol at ICRISAT-PQL for export
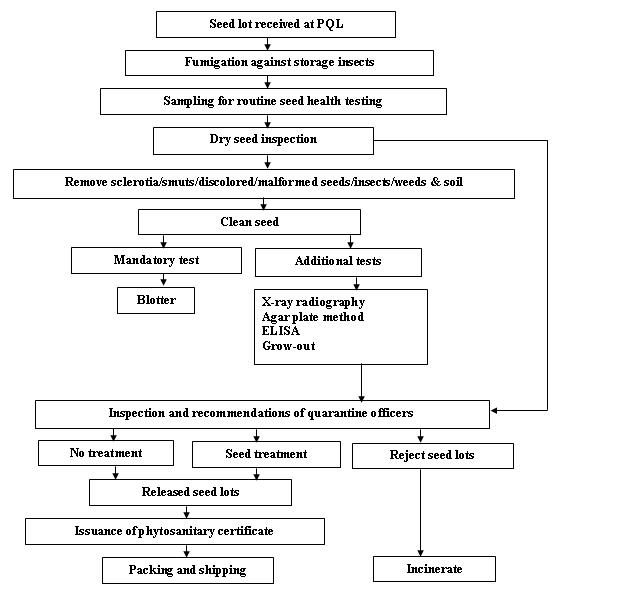
References and further reading
Chakrabarty, SK, K. Anitha, AG Girish, B Sarath Babu, RDVJ Prasad Rao, KS Varaprasad, PK Khetarpal and RP Thakur. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin 69. Rajendranagar 500 030, Andra Pradesh, India. National Bureau of Plant Genetic Resources, Pantacharu 502 324, Andra Pradesh, India. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT).
Fungi - pigeonpea
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Scientific name
Colletotrichum cajani Rangel.
Importance
High.
Significance
Anthracnose is destructive and infects leaves, pods and seeds especially during the period of heavy rainfall. The losses are mainly due to dropping of pods at young stage and discoloration and decay of some or all the seeds in the infected pods. In a harvest of affected crop, 86% pods have been found to be infected with 36% unmarketable seeds (Tucker 1927).
Symptoms
The typical symptoms are sunken spots on pods due to drying up and collapse of the cells in the center. The presence of pink sticky spore masses that develop during the moist weather have quite an ulcer like appearance. The main symptoms include spots on pods and leaves, blackening and shrinking of veins of infected leaves and their premature fall. The infected pods become distorted, abort and die. Affected young pods drop and all the seeds in the infected pods are discolored and decayed. Anthracnose spots appear as irregular, brown to grayish stem lesions containing dark, scattered acervuli. Severe infection leads to drying and death of infected branches. Minute spots with dark margins and gray centers scattered with acrvuli are also common on the leaves and pods of infected plants (Reddy et al. 1993).
 Anthracnose (Colletotrichum cajani) of pigeonpea: (A)leasions on plant and pods (photo: www.agrociencia-panama.blogspot.com); (B)lesion on stem (photo: ICRISAT). |
Hosts
Many ornamental plants and leguminaceous weeds.
Geographic distribution
The disease was first reported from Brazil in 1927 and subsequently from several other countries like Puerto Rico, India, USA, Venezuela and Zambia (Nene et al. 1996).
Biology and transmission.
The conidia are cylindrical, broadly elliptical or irregular with rounded ends and 12-17 ´ 3.5-7.2 m in size. The setae are numerous, and are curved, septate and 100 ´ 3.5 m in size. Appressoria apparently analogous to chlamydospores and were observed after 2 months on PDA and steamed pigeonpea pods. The pathogen has been associated with seeds of pigeonpea and is considered as the quarantine important pathogen (Nirula 1980).
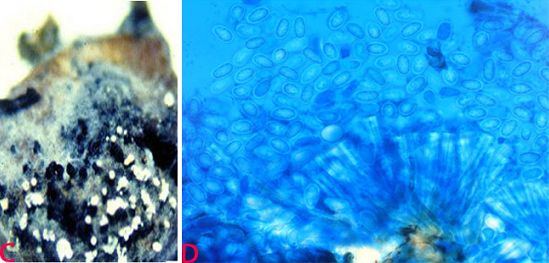
Detection/indexing methods at ICRISAT
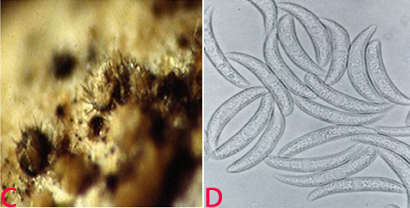 Anthracnose of pigeonpea:(C)acervuli on seed and (D)conidia. (photos: ICRISAT). |
- Pre-export field inspection and blotter test are used.
Treatment/control
- Rejection of the infected seed samples.
Procedures followed in case of positive test at ICRISAT
- Rejection of the infected seed samples.
References and further reading
Nene YL, Sheila VK, Sharma SB. 1996. A world list of chickpea and pigeonpea pathogens. 5th edition. Patancheru, Andhra Pradesh, India: ICRISAT, pp27.
Nirula KK. 1980. Quarantine to seed exchange at the ICRISAT. Seed Pathology News 13: 11-12.
Reddy MV, Raju TN, Sharma SB, Nene YL, McDonald D. 1993. Handbook of pigeonpea diseases. Information bulletin no. 42. Patancheru, AP, 502324, India: International Crops Research Institute for the Semi-Arid Tropics. 64pp.
Tucker CM. 1927. Pigeonpea anthracnose. Journal of Agricultural Research 34: 589-596.
Scientific name
Alternaria alternata (Fries) Keissler.
Other scientific names
Alternaria fasciculate, Alternaria rugosa, Alternaria tenuis, Macrosporium fasciculatum, Torula alternate.
Importance
Medium.
Significance
In the late sown pre-rabbi pigeon pea, Alternaria blight has been identified as potential problem in some states of India (Reddy et al. 1993).
Symptoms
Alternaria alternata causes circular necrotic spots on leaves that develop quickly forming typical concentric rings. The lesions appear on aerial plant parts including pods. They cause severe blightening of the leaves and defoliation and drying of infected branches (Reddy et al. 1993).
 Alternaria alternata of pigeonpea: (A)leaf spot; (B)leaf blight (photos: ICRISAT). |
Hosts
Over 380 hosts have been recorded in the USDA Systematic Mycology and Microbiology Fungus-Host Distribution Database. It is an opportunistic pathogen on numerous hosts causing leaf spots, rots and blights on many plant parts. It can also cause upper respiratory tract infections in AIDS patients and asthma in people with sensitivity.
Geographic distribution
Alternaria blight is distributed in India, Kenya and Puerto-Rico (Reddy et al. 1993).
Biology and transmission
Mycelium of the pathogen is usually light olive green to brown. Hyphae are dark brown, thick, septate and branched. Conidiophores are pale brown to olive brown, 25-60 ´ 3-3.5 µm in size and straight or flexuous. Individual conidiophores arise directly from substrate forming bushy heads consisting of 4-8 large catenate conidia chains. Secondary conidiophores are generally short and 1-celled. Conidia are pale brown to light brown, obclavate to obpyriform or ellipsoid, short conical beak at the tip, or sometimes beakless. Surface of conidia is smooth to verruculose, 20-63 ´ 9-18 µm in size. Conidia are septate with several (1-5) vertical and 3-8 transverse septa. The fungus sporulates well under warm and humid conditions (Kannaiyan and Nene 1977). Alternaria alternata has been found to be internally seedborne and the infection was detected in all parts of the seed (Girish et al. 2007).
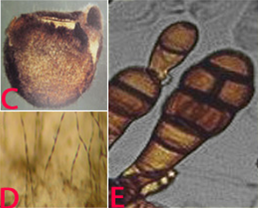 (C)fungal growth on seed; (D)conidia on seed and E. conidia (photos: ICRISAT). |
Detection/indexing methods at ICRISAT
- Pre-export field inspection and blotter test are used.
Treatment/control
- Seed treatment with the mixture of iprodione 25% + carbendazim 25% (1:1 by vol.) at 2 g a.i. kg-1 seed (Girish et al. 2007).
Procedures followed in case of positive test at ICRISAT
- Seed treatment with the mixture of benomyl + thiram (1:1) at 3 g kg-1 seed.
References and furter reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Girish AG, Sowjanya YV, Chakrabarty SK, Thakur RP. 2007. Studies on seedborne nature and management of Alternaria alternata and A. brassicae in pigeonpea. Indian Journal of Plant Protection 35: 128-130.
Kannaiyan J, Nene YL. 1977. Alternaria leaf spot of pigeonpea. Tropical Grain Legumes Bulletin 9:34.
Reddy MV, Raju TN, Sharma SB, Nene YL, McDonald D. 1993. Handbook of pigeonpea diseases. Information bulletin no. 42. Patancheru, AP, 502324, India: International Crops Research Institute for the Semi-Arid Tropics. 64pp.
Scientific name
Lasiodiplodia theobromae (pat.) Griffin & Maubl.
Other scientific names
Botryodiplodia ananassae, Botryodiplodia elasticae, Botryodiplodia theobromae, Botryodiplodia tubericola, Chaetodiplodia grisea, Diplodia ananassae, Diplodia theobromae, Lasiodiplodia nigra, Lasiodiplodia tubericola, Lasiodiplodiella triflorae, Macrophoma vestita.
Importance
Medium.
Significance
Lasiodiplodia theobromae affects a very wide range of crops, particularly at the postharvest stage. Infected seeds have reduced germination (Ellis et al. 1978).
Symptoms
Seed rotting and seedling blight.
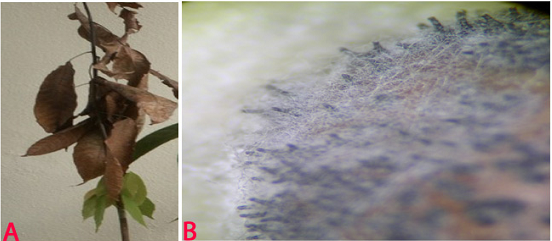 Blight (Lasiodiplodia theobromae) of pigeonpea: (A)lesions on plant (photo: www.bspporg.uk.com); (B) pycnidia on seed. (Photo: ICRISAT) |
Hosts
Citrus aurantiifolia (lemon), Theobroma cacao (cocoa), Arachis hypogaea (groundnut), Gossypium (cotton), Musa (banana), Mangifera indica (mango), Zea mays (maize), Allium spp. (onions, garlic, leek, etc.), Ananas comosus (pineapple), Capsicum annuum (bell pepper), Dioscorea (yam), Cajanus cajan (pigeon pea), Glycine max (soyabean), Ipomoea batatas (sweet potato), Manihot esculenta (cassava), Nicotiana tabacum (tobacco), Oryza sativa (rice), Saccharum officinarum (sugarcane), Sorghum bicolor (Sorghum), Vigna unguiculata (cowpea) and Vitis vinifera (grapevine).
Geographic distribution
Lasiodiplodia theobromae is world wide in distribution especially in Africa, Asia, Western hemisphere and Oceania.
 (C)black and white conidial ooze on seed and (D)single celled immature conidia (photos: ICRISAT). |
Biology and transmission
Colonies of the pathogen on oatmeal agar are grayish or mouse grey to black, fluffy with abundant aerial mycelium, reverse fuscous black to black. Pycnidia appear on the infected leaves, stems and fruits. Pycnidia are immersed and thick-walled, and aggregated in clusters immersed in a stroma frequently up to 5 mm wide, erumpent, often with a distillate papillate ostiole (Punithalingam 1976). Conidiophores are hyaline, simple, sometimes septate, rarely branched, cylindrical, arising from the inner layers of cells lining the conidiomatal cavity. Conidia are initially aseptate, hyaline, granulose, subovoid to ellipsoid-oblong, thick-walled, base truncate. Mature conidia are one-septate, cinnamon to fawn, often longitudinally striate, 18-30 ´ 10-15 mm. Paraphyses are hyaline, cylindrical, sometimes septate, up to 50 mm long. Seedborne nature of L. theobromae was reported in about 29% of the seeds of pigeonpeas (Richardson 1990).
Detection/indexing methods at ICRISAT
- Pre-export field inspection and blotter method
Treatment/control
- Seed treatment with a mixture of benomyl + thiram (1:1) at 3 g kg-1 seed.
Procedures followed in case of positive test at ICRISAT
- Seed treatment with a mixture of benomyl + thiram (1:1) at 3 g kg-1 seed.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Ellis MA, Paschal EH, Ravalo EJ, Rosaria E. 1978. Effect of growing location on internally seed-borne fungi, seed germination and field emergence of pigeon pea in Puerto Rico. J. Agric. Univ. P.R 62:355-360.
Punithalingam E. 1976. Botryodiplodia theobromae, No. 519 In: Descriptions of Pathogenic Fungi and Bacteria. Wallingford, UK: CAB International.
Richardson MJ. 1990. An Annotated List of Seed-borne Diseases. 4th edn. Zurich, Switzerland: International Seed Testing Association.
Nematodes - pigeonpea
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
 Pearly root (Heterodera cajani) of pigeonpea symptoms in field (photo: ICRISAT). |
Pearly root
Scientific name
Heterodera cajani Koshy.
Other scientific name
Heterodera vigni.
Importance
Low.
Significance
Yield losses of over 30% and 28% reduction in plant growth have been reported (Sharma et al. 1993).
Symptoms
Heterodera cajaniis primarily a root-parasite. The most important characteristic symptom is the presence of cysts on the root surface. Identification of "pearly root" caused by the presence of white females is a useful symptom of H. cajani infestation in pigeon pea at the vegetative stage (Sharma 1993). The symptoms of nematode injury include stunting, reduced leaf lamina size and yellowing on cotyledonary leaves (Gaur and Singh 1977). Flowers and pods are reduced in size and number and the root system may also be poorly developed. Foliage symptoms are generally not apparent even in heavily infested soils, but a reduction in height and vigor of the infected plants can be discerned by careful comparison with healthy plants.
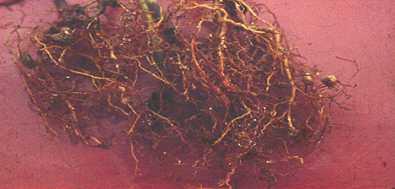 Root damage symptoms of pearly root of pigeonpea |
Hosts
Cajanus cajan (pigeon pea), Cyamopsis tetragonoloba (cluster bean), Lablab purpureus (hyachinth bean), Vigna aconitifolia (aconite-leaved kidney beans), Vigna radiata (bean, mung), Vigna mungo (black gram), Phaseolus vulgaris (common bean), Pisum sativum (pea), Sesamum indicum (sesame) and Vigna unguiculata (cowpea) (Sharma and Nene 1985).
Geographic distribution
Heterodera cajani has been reported from Egypt, India and Pakistan. It is wide spread in sandy-loam soils in northern India and in black cotton soils in western and southern India (Reddy et al. 1993; Varaprasad et al. 1997).
Biology and transmission
The adult females are lemon-shaped and sedentary in habit, and remain attached to roots semi-endoparasitically. These are white to slightly brown, with a neck and posterior cone-like elevation on which the vulva is situated; turns into a cyst of same size and shape. Posterior part of body protruding outside the root usually appears with small rounded egg sac attached to it. Cephalic region has two annules; the second is larger than the first. Stylet is of medium strength, in two equal parts; basal knobs round to slightly anteriorly flattened. Median esophageal bulb large rounded, with well developed valve plates. The excretory pore is placed posterior behind the median bulb. Oesophageal glands extend over the intestine. Ovaries are paired and convoluted. Uterus with several eggs filling most of the body. Vulva is a large transverse slit on a cone-shaped elevation of the body. Anus is close to vulva. An egg sac is present. The size of egg sacs varies between 0.5-2 times the sizes of cyst. Egg sacs are yellow, occasionally purple. Few to 200 eggs (average 54) are found in the egg sacs (Koshy and Swarup 1971). Eggs may be retained inside the female body but many are laid in a gelatinous matrix forming egg sacs. Eggs are oval, 95-115 µm long, and 37-48 µm wide. Eggshell is hyaline, without surface markings. Morphological characteristics of 2nd-, 3rd- and 4th-stage juveniles of H. cajani, H. avenae and H. mothi are described and compared by Taya and Bajaj (1986). Males are vermiform.
Detection/indexing methods at ICRISAT
- Pre-export field inspection.
Treatment/control
- Not available.
Procedures followed in case of positive test at ICRISAT
- Rejection of the seed samples.
References and further reading
Gaur HS, Singh I. 1977. Pigeon-pea cyst nematode, Heterodera cajani, associated with the moong crop in the Punjab State. Journal of Research, Punjab Agricultural University 14: 509.
Koshy PK, Swarup G. 1971. Investigations on the life history of the pigeon-pea cyst nematode, Heterodera cajani. Indian Journal of Nematology 1:44-51.
Reddy MV, Raju TN, Sharma SB, Nene YL, McDonald D. 1993. Handbook of pigeonpea diseases. Information bulletin no. 42. Patancheru, AP, 502324, India: International Crops Research Institute for the Semi-Arid Tropics. 64pp.
Sharma SB. 1993. Pearly root of pigeonpeas caused by Heterodera cajani. Indian Journal of Nematology 21: 169.
Sharma SB, Nene YL. 1985. Additions to host range of pigeonpea cyst nematode, Heterodera cajani. International Pigeonpea Newsletter 4: 42.
Sharma SB, Nene YL, Reddy MV, McDonald D. 1993. Effect of Heterodera cajani on biomass and grain yield of pigeon pea on vertisol in pot and field experiments. Plant Pathology 42: 163-167.
Taya AS, Bajaj HK. 1986. Larval stages of Heterodera avenae Woll, H. cajani Koshy and H. mothi Khan and Husain. Indian Journal of Nematology 16: 160-162.
Varaprasad KS, Sharma SB, Loknathan TR. 1997. Nematode constraints to pigeonpea and chickpea in Vidarbha region of Maharashtra in India. International Journal of Nematology 7: 152-157.
Bacteria - pigeonpea
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Bacterial blight
Scientific name
Pseudomonas syringae pv. pisi (Sackett) Young et al.
Other scientific name
Pseudomonas pisi.
Importance to CGIAR centers
Medium.
Significance
Severe infection and substantial crop losses have been reported from winter-sown peas in southern France, New Zealand and South Africa (Boelema 1972; Taylor 1972). The disease as such, however, does not appear to be of great economic importance in Europe.
Symptoms
Symptoms usually appear on the stem near the soil as water-soaked and later olive-green to purple-brown spots. The infection extends upwards to the stipules and leaflets, where veins turn brown to black and adjacent tissues become diseased in a fan-like pattern. The interveinal tissues may become water soaked and then yellowish to brown, finally drying out and becoming papery. Lesions on leaflets and pods begin as small, round, oval or irregular dark-green water-soaked spots at first, and later enlarge and coalesce but are sharply defined by the veins. Cream-colored bacterial ooze may be found on the lesion surface that, on drying, gives a glossy appearance. The leaflets later become yellowish and the spots brown and papery. Ripening pods become twisted and dry, lesions on them are sunken and greenish-brown. Lesions on the pod may be limited to a narrow band on the sutures. Infected seeds show a water-soaked spot near the hilum and/or are shrivelled, with a brown-yellow discoloration. Infection often takes place on sepals, spreading to the flowers, and flower buds may be killed before they open.
Hosts
Pisum sativum var. arvense (peas) is the principal host. Natural infection has also been recorded on Lablab purpureus (hyacinth bean), Lathyrus latifolius (Everlasting-pea or sweet-pea Everlasting), L. odoratus (sweet pea) and Vicia benghalensis (purple vetch).
Geographic distribution
Bacterial blight is world wide in distribution.
Biology and transmission
Pseudomonas syringae pv. pisi is a motile, gram-negative, non-spore-forming rod, 0.7 ´ 2-3 µm, with one to five polar flagella. Disease has been observed to develop more readily on soils with high moisture content. Infections can occur through contact of diseased and healthy foliage and insects may have a role in transmission. The bacterium can survive on or within seeds for at least 10 months (Skoric 1927) and for several months on diseased plant debris in the field (Harris 1964).
Detection/indexing methods at ICRISAT
- Detection of the pathogen in seeds is usually performed by soaking ground pea seeds in buffer at low temperature (4°C) for 4-16 h and subsequent isolation from the (centrifuged) soaking solution. For isolation from diseased tissues and seeds, King's medium B can be used, with supplement of boric acid, cephalexin and cycloheximide if necessary (Mohan and Schaad 1987).
Treatment/control
- Not known since it is not detected at ICRISAT.
Procedures followed in case of positive test at ICRISAT
- Incineration of the infected crop and rejection of the seed samples.
EPPO protocols
EPPO (OEPP/EPPO 1990) recommends that pea seeds should come from a field or area free from P. syringae pv. pisi, or else that the seed crop should have been inspected. However, seed-testing techniques are now available to detect the bacteria.
References and further reading
Boelema BH. 1972. Bacterial blight (Pseudomonas pisi Sackett) of peas in South Africa, with special reference to frost as a predisposing factor. Mededelingen Landbouwhogeschool Wageningen 72: 1-87.
Harris DE. 1964. Bacterial blight of peas. Journal of Agriculture, Victoria Department of Agriculture 62: 276-280.
Mohan SK, Schaad NW. 1987. Semiselective agar medium for isolating Pseudomonas syringae pv. syringae and pv. phaseolicola from bean seed. Phytopathology 77: 1390-1395.
OEPP/EPPO. 1990. Specific quarantine requirements. EPPO Technical Documents No. 1008.
Skoric V. 1927. Bacterial blight of peas; overwintering, dissemination and pathological histology. Phytopathology 17: 611-628.
Taylor JD. 1972. Races of Pseudomonas pisi and sources of resistance in field and garden peas. New Zealand Journal of Agricultural Research 15: 441- 447.




