stog-chickpea
Weeds - chickpea
Contributors to this page: ICRISAT, India (RP Thakur, AG Girish, VP Rao); ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
|
Contents: |
Scientific names
Orobanche foetida Pers.
Orobanche crenata Forsk.
Orobanche ramosa L.
Significance
Yield reductions of 30 to 70% are not uncommon. International markets may reject broomrape-contaminated produce.
Symptoms
The appearance of the parasite itself is the clearest sign of infestation. The parasite has erect, branched or unbranched aerial flowering shoots. The leaves are reduced to scales and are disposed alternatively. The shoots bear bisexual tubular flowers in a spike. The tiny seeds are produced in enormous numbers in capsules.
Hosts
Broomrapes usually only parasitic to broadleaved plants but there are a few records of attacks on monocots (grasses, etc.). Branched Broomrape attacks many crops and common weeds. Non-host crops can support infestations because of their weed component, and the produce of such crops may carry Broomrape seeds. Some Broomrape species have specialized biotypes or races. Plants of the same species attack different sets of hosts in different geographic areas. Susceptibility to attack also depends on the cultivar or genetic characteristics of a particular crop. The plants attacked by Branched Broomrape around the world include:
Crops: bean, broad bean, cabbage, capsicum (peppers), canola, carrot, cauliflower, celery, chickpea, clovers, eggplant (aubergine), flax, hemp (cannabis), hops, lentil, medicagos, onion, parsnip, paprika, pea, pyrethrum, sunflower, tobacco, tomato and potato.
Geographic distribution
Western and Central Asia (especially in Afghanistan, Syria, Egypt, India, Iran, Jordan, Lebanon, Morocco, Pakistan, Saudi Arabia, Australia, Europe (especially the Mediterranean), N. africa and N. america.
Biology and transmission
Seeds germinate in moist soil at 18 to 23°C and require moist soil for one week to become established. The roots parasite the roots of host plants for 40 to 50 days. Stems emerge in spring and are visible for only about three weeks from the end of September through to October. Flowering is rapid, within 6 to 9 days after stem emergence, with a similar period between the cessation of flowering and the ripening of fruit. An infestation may contain many plants that do not produce above-ground parts.
Dispersal from property to property and within properties in the infested area in South Australia appears to be mainly by carriage on vehicles and machinery. Seeds stick to equipment and animals that contact the plant and can also be dispersed by wind and water (including irrigation water/sprinklers), in animal manure, soil, fodder, on livestock and on footwear and clothing. Seeds attach to crop seeds and other produce. Seedlings imported from infested areas can carry seeds stuck to their leaves or in the soil. Seeds remain viable after passage through the digestive tracts of animals including cattle and goats.
Detection/indexing method in place at the CGIAR Centres
- At ICARDA - Filter Wash Test.
- At ICRISAT - Not applicable.
Treatment/control
Hand weed shoots prior to their seed formation to reduce inoculum levels. This is recommended in minor infestations only.
- Sow the crop late to help plants escape the worst effects of infection.
- Plow deep to bury Orobanche seeds into deeper soil layers to reduce infestation.
- Use selective herbicides such as imazaquin (10-15 ml a.i./ha).
- Use soil solarization to reduce soil-borne inoculum.
- Use resistant varieties, if available.
Procedure followed at the CGIAR Centres in case of positive test
- At ICARDA - Mechanical rotation, herbicides.
- At ICRISAT - Not applicable.
References and further reading
http://www.agroatlas.ru/en/content/weeds/Orobanche_ramosa/
|
Orobanche foetida (photo: ICARDA) |

Orobanche crenata (photo: ICARDA) |

Orobanche ramosa (photo: ICARDA) |

Orobanche egyptiaca (photo: ICARDA) |
Scientific name
Cuscuta hyalina Roth.
Other scienfiic name
Cuscuta pentagona.
Significance
The effect of field dodder on 12 legume crops was investigated in a greenhouse experiment. The legume crops showed great variations in response to field dodder parasitism. Based on the reduction in host dry weight (biological yield) caused by the parasite, chickpea lost >50% of its biological yield and was considered highly susceptible.
Symptoms
The appearance of the parasite itself is the best diagnostic symptom. It has fine, yellow or orange, thread-like branches which grow and entwine around the stems and other aboveground parts of the host.
Dodder-infected areas appear as patches in the field and continue to enlarge during the growing season. In late spring and early summer, dodder produces a massed cluster of white flowers.
Infected host plants become weakened, decline in vigour and produce poor yields.
Several patches might coalesce to form large areas that can be easily recognized by the yellowish colour of the parasite strands.
Hosts
Cuscuta species attack a wide range of species including vegetables, fruits, ornamentals and woody plants. It is reported as a weed in 25 crops in 55 countries.
Geographic Distribution
Cosmopolitan.
Detection/indexing method in place at the CGIAR Centres
- At ICARDA - Filter Wash Test.
- At ICRISAT - Not applicable.
Treatment/control
- Cultural Practices: Dodder is best controlled through prevention by using clean seed. Ornamental beds should be kept clean of seed-producing plants. Once dodder becomes established, eradication is almost impossible. Mechanical removal can be attempted, but once dodder has produced the haustoria it is almost impossible to avoid damage to the host plant. If dodder has germinated and has attached to a host plant, removal of both is recommended to eradicate.
- Herbicide Use: use of pre-emergent herbicides is the best chemical approach. These products must be applied prior to the germination of the seed as the small seedling root will drop off once the haustoria has formed. Dinitroanaline herbicides have been reported to be effective. Dichlobenil has also shown good results. Read all labels to ensure that the herbicides can be used around the host plants. Glyphosate has shown results as a postemergent herbicide, but its use is limited as it will also damage the host plant. Phenoxy herbicides have only shown limited control and again, injury to the host plant can occur.
Procedure followed at the CGIAR Centres in case of positive test
- At ICARDA - Mechanical control, cultural practices and herbicides.
- At ICRISAT - Not applicable.
References and further reading
http://www.icarda.org/Publications/Field_Guides/Lentil/Lentil.htm#Lent7.Html
http://www.weedalert.com/weed_pages/wa_dodder.htm

Cuscuta hyalina (photo: ICARDA) |

Cuscuta campestris (photo: ICARDA) |
Viruses - chickpea
Contributors to this page: ICRISAT, India (RP Thakur, AG Girish, VP Rao); ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
|
Contents: |
Scientific Name
Pea Seedborne Mosaic Virus (PSbMV).
Other scientific names
Pea fizzle top virus, pea leaf rolling virus, pea leaf roll mosaic virus, pea leaf rolling mosaic virus.
Significance
PSbMV is of economic importance in pea, faba bean and lathyrus, mainly due to its effect on seed quality. It has been mistakenly considered to be a minor disease because it often causes only minor yield loss and mild symptoms. However, at the International Centre for Agriculture in the Dry Areas (ICARDA), Syria, glasshouse studies on yield losses due to PSbMV in chickpea, faba bean, lentil and pea showed losses of 66%, 40.5%, 44.6% and 49.2% respectively. Surveys of pulse crops over a number of years showed that up to 15% of crops were infected with PSbMV, with a within crop incidence of up to 5% of plants. In surveys in WA in 1999, PSbMV was found in 42% of field pea crops and the level of virus detected in pea seed stocks was up to 63%. The surveys in WA indicated that PSbMV has a severe effect on seed quality of pulse crops with seed coat symptoms evident on >80% of faba bean, >50% of lathyrus species and field peas and 5% of chickpea seed.
Symptoms
The disease is characterized by a mild mottling on the leaves.
The affected plants are reduced in growth, which is easily recognized, especially when they are compared with healthy plants. Yield reductions of 15-16% could occur in some different cultivars. Seeds from infected plants occasionally show dark staining on the seed.
Plants may show no symptoms or there may be chlorosis in new shoots, mottling on leaves, shoot tip necrosis and stunting of plants.
 PSbMV (photo: www1.agric.gov.ab.ca) |
Hosts
The natural host range of PSbMV is limited to the Fabaceae. It infects temperate pulses (chickpea, faba bean, field pea, lentil) other legumes (garden pea, narbon bean) and pastures (lathyrus and vetch). A number of PSbMV pathotypes have been recognised by their ability to infect a number of pea differential genotypes.
Geographic distribution
Cosmopolitan.
Biology and transmission
The virus is believed to have spread worldwide through the exchange of infected seed. Seed transmission rates of up to 100% in peas and up to 44% in lentils have been reported. In Victoria, we have detected PSbMV at low levels in some commercial chickpea seedlots (0.4% of seed) and at higher levels in field pea and lentil seedlots (greater than 2% of seed). In the USA, 3% PSbMV infection in pea seedlots and 32-40% in lentil seedlots have been reported. At ICARDA, PSbMV was found to be transmitted through lentil seeds at rates of up to 44%. PSbMV is also transmitted in a non-persistent manner by more than 20 aphid species and by mechanical means. The most efficient vector is the pea aphid (Acyrthosiphon pisum). The other species are as follows: A. pelargoni, A. sesbaniae, Aphis craccivora, A. fabae, A. gossypii, A. nasturtii, Aulacorthum circumflexum, A. solani, Brevicoryne brassicae, Cryptomyzus ribis, Dactynotus escalantii, Macrosiphum avenae, M. euphorbiae, M. pisi, M. rosae, Metopolophium dirhodum, Myzus persicae, Ovatus crataegarius, Phorodon cannabis, Rhopalosiphum padi and Semiaphis dauci. In Victorian pulse crop surveys, we have found cowpea aphid (Aphis craccivora), foxglove aphid (Aulacorthum solani) and green peach aphid (Myzus persicae), which are all vectors of PSbMV.
Detection/indexing method in place at the CGIAR Centres
- At ICARDA - Not important. TBIA, Enzyme-linked immunosorbent assay (ELISA), Tissue blot immunoassay (TBIA).
- At ICRISAT - Not applicable.
Treatment
- Seed is considered to be the main source of PSbMV, therefore sowing virus tested seed is the most effective way of controlling this virus. Commercial seed tests are available.
- Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses, such as PSbMV. Pulse crops should be sown away from other legumes to minimize the spread of PSbMV.
References and further reading
http://www.icarda.org/Publications/Field_Guides/Lentil/Lent7.Html
http://image.fs.uidaho.edu/vide/descr575.htm
http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/prm7819
http://www.inra.fr/hyp3/pathogene/6psbmov.htm
Scientific Name
Alfalfa Mosaic Virus (AMV).
Other scientific names
Lucerne Mosaic Virus, Potato Calico Virus.
Significance
The studies showed that up to 17% of crops were infected with AMV, with a within-crop incidence of up to 21% of plants.
Symptoms
Chlorosis of the terminal bud and twisting, followed by necrosis and the subsequent proliferation of secondary branches. Such new secondary branches are stiff and erect with smaller leaflets that show a mild mottle.
Very few pods are produced.
Terminal bud necrosis can also be caused by iron deficiency but proliferation of branches is not seen in iron deficient plants.
Hosts
The host range of AMV is wide and not limited to Fabaceae. Temperate pulse hosts include chickpeas, faba beans, field peas, lentils, narbon beans, grass peas and vetch. Pasture legume hosts include lucerne, burr medic and other annual medics and a number of clover species.
Geographic distribution
Algeria, India, Iran, Morocco, New Zealand and USA.
Biology and transmission
The virus is transmitted by a vector; transmitted by mechanical inoculation; transmitted by grafting; not transmitted by contact between hosts; transmitted by seeds (50% in alfalfa seeds from individual infected plants and up to 10% in commercial seed, transmitted by pollen to the seed).
Vector Transmission
The virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; Myzus persicae and at least 13 other species. The virus is transmitted in a non-persistent manner.
Detection/indexing method in place at the CGIAR Centres
- At ICARDA- Not important. Enzyme-linked immunosorbent assay (ELISA), Tissue blot immunoassay (TBIA).
- At ICRISAT- Not applicable.
Treatment
- Chemical control of aphids is not an effective method for controlling AMV.
- Sowing healthy seed, managing weeds and other cultural practices to minimize AMV spread are recommended.
- Growing crops adjacent to infected lucerne or pasture will increase the risk of crop infection.
Procedure followed at the CGIAR Centres in case of positive test
No specific procedures notified.
References and further reading
http://www.icrisat.org/vasat/learning_resources/chickpea/chickpea_diseases/viral_mosaic.htm
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.010.0.01.001.htm
 |
 |
 |
|
AMV (photos: www.dpi.vic.gov.au) |
||
Bean Yellow Mosaic, Narrow leaf
Scientific Name
Bean Yellow Mosaic Virus (BYMV).
Other scientific names
Bean Virus 2, Canna Mosaic Virus.
Significance
BYMV is sometimes found in faba bean crops and occasionally in peas with within crop virus incidences of 1-15% and up to 7% respectively.
Symptoms
Yellowing and drying of the plants with feathery and deformed leaves are common.
The leaves below the proliferated branches turn yellow, show interveinal chlorosis, or mosaic depending on the genotype tested. The overall height of the plant is reduced.
Affected plants produce very few, distorted flowers that develop into very small pods.
The seeds from infected plants are black, small, and shriveled.
 BYMV (photo: www.icrisat.org) |
Hosts
The host range of BYMV is wide and not limited to Fabaceae. The virus is reported to infect nearly 200 species in 14 families. Temperate pulse hosts include chickpeas, faba beans, field peas, lentils and lupins. Temperate legume pasture hosts include lathyrus, lucerne, vetch and medic and clover species. BYMV has a number of subtropical and tropical pulse hosts, including soybeans, peanuts and French beans as well as legume pasture hosts. It also infects ornamental hosts, the most common being gladiolus species.
Geographic distribution
The virus is probably distributed worldwide.
Biology and transmission
The virus is transmitted by a vector. The virus is transmitted by mechanical inoculation; and transmitted by seeds up to 3%.
Vector Transmission:
Virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; more than 20 ssp. including Acyrthosiphon pisum, Macrosiphum euphorbiae, Myzus persicae, Aphis fabae. Virus is transmitted in a non-persistent manner.
Detection/indexing method in place at the CGIAR Centres
- At ICARDA - Not important. Enzyme-linked immunosorbent assay (ELISA), Tissue blot immunoassay (TBIA).
- At ICRISAT - Not applicable.
Treatment
- Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses such as BYMV.
- Pulse crops should be sown away from legume pastures to minimize the spread of BYMV.
- The spread of virus can also be reduced by controlling weed hosts from in and around paddocks.
Procedure in place at the CGIAR Centres in case of positive test
No specific procedures notified.
References and further reading
http://www.icrisat.org/vasat/learning_resources/chickpea/chickpea_diseases/viral_narrowleaf.htm
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.057.0.01.009.htm
Cucumber Mosaic, Proliferation
Scientific Name
Cucumber Mosaic Virus (CMV).
Other Scientific Names
Banana Infectious Chlorosis Virus, Cleus Mosiac Virus.
Significance
In field experiments infection in chickpea was shown to diminish shoot dry weight by 72-81%, seed yield by 80-90% and individual seed yield by 17-25%.
Symptoms
Bushy and stunted plants.
Diseased plants produce few flowers and pods, and many die prematurely.
 CMV (photo: ICARDA) |
Hosts
Susceptible host species are found in the families: Amaranthaceae, Apocynaceae, Chenopodiaceae, Compositae, Convolvulaceae, Cruciferae, Cucurbitaceae, Leguminosae-Papilionoideae, Malvaceae, Phytolaccaceae, Polygonaceae, Scrophulariaceae, Solanaceae, Tetragoniaceae, Tropaeolaceae, Umbelliferae.
Geographic distribution
The virus is probably distributed worldwide.
Biology and transmission
The virus is transmitted by a vector; the virus is transmitted by mechanical inoculation; it is transmitted by seeds (in 19 species but in variable extents).
Vector Transmission
The virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; more than 60 subspecies including Acyrthosiphon pisum, Aphis craccivora and Myzus persicae. The virus is transmitted in a non-persistent manner.
Detection/indexing method in place at the CGIAR Centres
- At ICARDA - Not important. Enzyme-linked immunosorbent assay (ELISA), Tissue blot immunoassay (TBIA).
- At ICRISAT - Not important.
Treatment
- Chemical control of aphids is not an effective method for controlling CMV.
- Sowing healthy seed, managing weeds and other cultural practices to minimize CMV spread are recommended.
- Growing crops adjacent to infected lucerne or pasture will increase the risk of crop infection.
Procedure followed at the centers in case of positive test
No specific procedures notified.
References and further reading
http://www.icrisat.org/vasat/learning_resources/chickpea/chickpea_diseases/viral_proliferation.htm
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.010.0.04.001.htm
Best practices for safe transfer of chickpea germplasm
Contributors to this page: ICRISAT, India (RP Thakur, AG Girish, VP Rao).
Best practices in place at ICRISAT
For the most recent practices relating to phytosanitary standards and protocols used at ICRISAT to test seed germplasm see the following reference:
Chakrabarty, SK, K. Anitha, AG Girish, B Sarath Babu, RDVJ Prasad Rao, KS Varaprasad, PK Khetarpal and RP Thakur. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin 69. Rajendranagar 500 030, Andra Pradesh, India. National Bureau of Plant Genetic Resources, Pantacharu 502 324, Andra Pradesh, India. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT).
Seed health testing protocol at ICRISAT-PQL for import
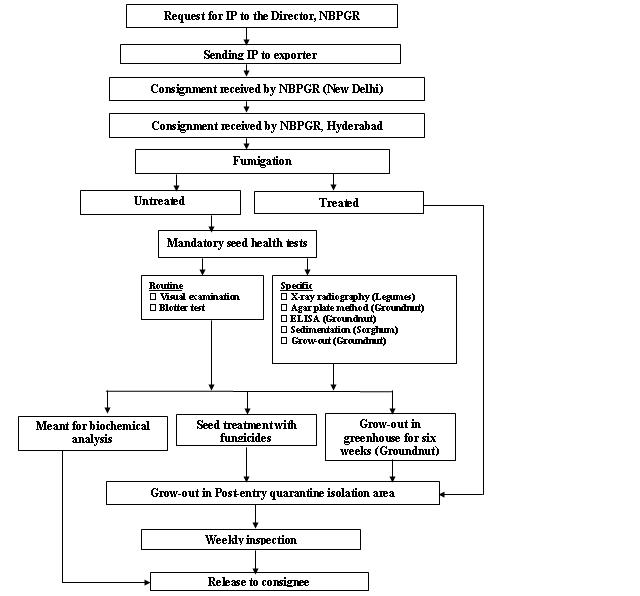
Seed health testing protocol at ICRISAT-PQL for export
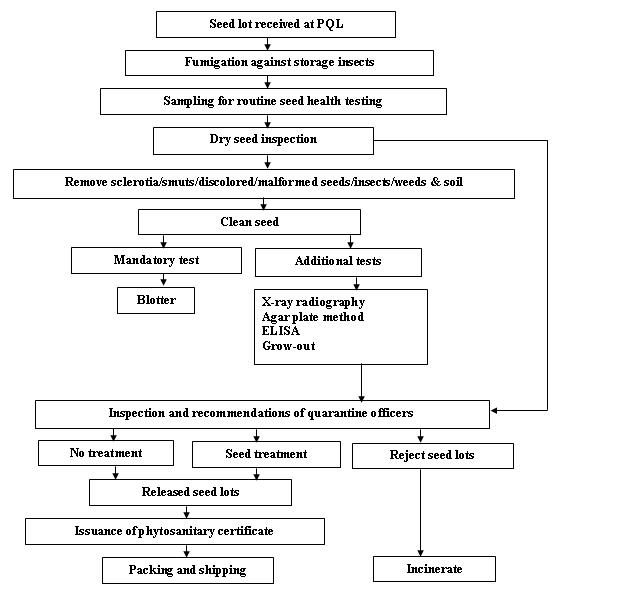
Insects - chickpea
Contributors to this page: ICRISAT, India (RP Thakur, AG Girish, VP Rao); ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
|
Contents: |
Scientific name
Callosobruchus analis Fabricius.
Other scientific names
Bruchus analis, Bruchus glaber, Bruchus jekelii, Bruchus obliquus, Bruchus ciceri,
Callosobruchus glaber, Callosobruchus jekelii.
Importance
Medium.
Significance
Infested chickpea lose their viability and are unfit for human consumption. In Africa, Asia, and Oceania, C. analis is considered a pest of economic importance for stored-legume grains (Southgate 1979).
Symptoms
Chickpea pods are seldom infested in the field. The pests attack nearly mature and dried pods during storage. The round exit hole and the white eggs on the pod wall are clearly visible. Infested stored seed can be recognized by the eggs on the seed surface and the round exit holes with the ‘flap’ of seed coat.
|
Stored Grain Pest (photo:www.invasive.org) |

Damaged grains with white eggs and floppy windows (photo: ICRISAT) |
Hosts
Cicer arietinum (chickpea), Cajanus cajan (pigeonpea) and other grain legumes.
Geographic distribution
Callosobruchus analis is widespread in Asia.
Biology and transmission
Adults are small, 3 mm long brown beetles with black spots on the elytra. Eggs are laid on the seed surface. Larvae feed and pupate entirely within the seed. One generation is completed in 4-5 weeks (Ranga Rao and Shanower 1999).
Detection/indexing methods used in CGIAR
- At ICRISAT - Dry seed examination and X-ray radiography are used for detection of the bruchides.
- At ICARDA - Visual and microscopic inspection.
Treatment/control
-
At ICRISAT
- Fumigation with methyl bromide by 32 g/m3 for 4 hrs followed by seed treatment with chlorpyriphos 3 g/kg-1 seed (Ghanekar et al. 1996).
-
At ICARDA
- Cleanliness in storage: stores should be cleaned from all residues of grains, straw and flour and be de-infested by spraying walls with malathion,
- Sacks, thresher and transportation vehicles also should be cleaned.
- Seeds stored for food/feed can be fumigated with phospine (Phostoxine). Seeds stored for planting can be treated with insecticides such as Actellic at 4-10 ppm a.i. (0.5 g/kg seed) or malathion at 10 ppm a.i., which will protect the seeds for several months.
Procedures in CGIAR in case of positive test
- At ICRISAT - Removal of the infested seeds followed by the seed treatment with chloropyriphos at 3 g/kg-1 seed.
- At ICARDA - Fumigation.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Ranga Rao GV, Shanower TG. 1999. Identification and Management of Pigeonpea and Chickpea Insects Pests in Asia. Information Bulletin No. 57, Patancheru, 502 324, A.P., India: International Crops Research Institute for the Semi Arid Tropics. 96pp.
Southgate BJ. 1979. Biology of the Bruchidae. Annual Review of Entomology 24:449-473.
Cowpea seed beetle, Adzuki bean seed beetle
Scientific names
Callosobruchus maculatus (Fabricius), 1775;
and
C. chinensis (L.), 1758.
Significance
The Callosobruchus beetles are principally a serious pest of products stored under hot dry conditions; complete destruction of grain and pulses may occur in a short time. In humid climates, the its competitors are so much greater that it has difficulty in establishing itself.
Symptoms/damage
At the larval stage the beetles tunnel into and develop within the beans. They may consume nearly the entire bean content. Pupation occurs within the beans and adults emerge through a round hole in the seed coat. Damage is caused by a combination of the feeding and contamination.
Hosts
The Callosobruchus beetles attack several legumes in storage, chickpea (Cicer arietinum), arhar (Cajanus cajan), green gram (Vigna radiata), pea (Pisum sativum) and kidney bean (Phaseolus vulgaris) seeds. On lentil, C. chinensis is the most common.
Geographic distribution
Both species are widespread and found in all continents with subtropical or tropical conditions (USA, Mediterranean, India and Australia).
Biology and transmission
The eggs are glued to the bean or the pod. On hatching the larvae bores into the seed where it makes a translucent 'window' in the seed before pupating. The larval and pupal stages are spent inside the bean. The adult emerges through the 'window' leaving a neat round hole. Infestations can begin in the field. Adults move to bean fields from trash beans left in sacks, harvesters, planters, or feed areas. The cowpea weevil readily attacks dried beans; thus this weevil can be a serious storage pest. Bean weevil infestations can also start in the field and may also originate from trash beans. As with the cowpea weevil, the bean weevil will attack dried beans and can be a serious pest in stored beans. Broad bean weevil infestations also start in the field, but this pest is not a storage problem.
Detection/indexing methods in place at the CGIAR Centres
- At ICRISAT - Dry seed examination and X-ray radiography are used for detection of the bruchides.
- At ICARDA -Visual and microscopic inspection.
Treatment/control
-
At ICRISAT
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos 3 g/kg-1 seed.
-
At ICARDA
- Cleanliness in storage: stores should be cleaned from all residues of grains, straw and flour and be de-infested by spraying walls with malathion; sacks, thresher and transportation vehicles should also be cleaned.
- Seeds stored for food/feed can be fumigated with phospine (Phostoxine). Seeds stored for planting can be treated with insecticides such as Actellic at 4-10 ppm a.i. (0.5 g/kg seed) or malathion at 10 ppm a.i., which will protect the seeds for several months.
Procedures in place within the CGIAR Centres in case of positive test
- At ICRISAT - Removal of the infested seeds followed by seed treatment with chloropyriphos at 3 g/kg-1 seed.
- At ICARDA - Fumigation.
References and further reading
http://www.icarda.org/Publications/Field_Guides/Lentil/Lentil.htm#Lent7.Html
http://www.infonet-biovision.org/default/ct/82/pests
http://agspsrv34.agric.wa.gov.au/Ento/pestweb/Query1_1.idc?ID=-1771861620
http://www.zin.ru/Animalia/coleoptera/rus/calmacdk.htm
http://www.infonet-biovision.org/default/ct/82/pests
 |
 |
|
|
Callosobruchus maculatus (photo: infonet-biovision.org) |
||
Scientific name
Trogoderma granarium Everts.
Other scientific names
Trogoderma afrum, Trogoderma khapra, Trogoderma quinquefasciata.
Importance
High.
Significance
Trogoderma granarium is a serious pest of stored products under hot dry conditions. Established infestations are difficult to control because of the beetle's ability to live without food for long periods of time and to survive on foods of low moisture content, its habit of crawling into tiny cracks and crevices and remaining there for long periods, and its relative tolerance to many surface insecticides and fumigants.
Symptoms
The Khapra beetle is one of the world's most feared stored product pests. The obvious signs of a Khapra beetle infestation are the larvae and cast skins. Larvae and adults are best identified by microscopic examination. Larvae are most likely to be seen just before dusk, since they are more active at that time (Anonymous 1981).
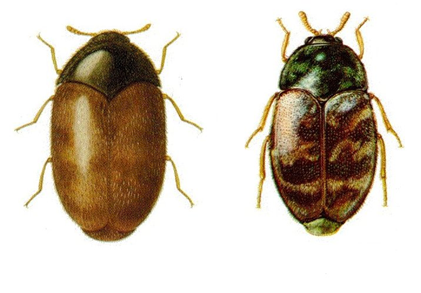 Khapra beetle (from: agspsrv34.agric.wa.gov.au) |
Host
Larvae feed on a wide variety of stored products and dried foods. They prefer whole grain and cereal products such as Triticum aestivum (wheat), Hordeum vulgare (barley), and Oryza sativa (rice), but larvae have been recorded on the following: Avena spp. (oats)), Secale spp. (rye), Zea mays (corn), dried blood, dried milk, fishmeal, Arachis hypogea (ground nuts), flour, bran, malt, Linum usitatissimum (flax seed), Medicago sativa (alfalfa seed), Lycopersicum esculantus (tomato seed), Phaseolus vulgaris (pinto beans), Vigna unguiculata (blackeyed cowpeas), Sorghum bicolor (sorghum seed) and many other food products (Lindgren and Vincent 1959; Lindgren et al. 1955).
Geographic distribution
The distribution of khapra beetle extends from Mayanmar (Burma) to West Africa and is limited by the 35° parallel to the north and the equator to the south. It has been introduced by commerce into some areas of similar climatic conditions (Anonymous 1981). The Khapra beetle is found in all continents where grain and grain products are stored.
Biology and transmission
The adults are oblong-oval beetles, approximately 1.6 to 3.0 mm long and 0.9 to 1.7 mm wide. Males are brown to black with indistinct reddish-brown markings on elytra. Females are slightly larger than males and lighter in colour. The head is small and deflexed with a short 11-segmented antenna. The antennae have a club of three to five segments, which fit into a groove in the side of the pronotum. The adults are covered with hairs. The eggs are milky white, turning pale yellow with age, cylindrical, 0.7 0.25 mm, one end rounded, the other pointed and bearing spine-like projections. Larvae are uniformly yellowish-white, except head and body hairs that are brown. As the larvae increase in size, their body colour changes to a golden or reddish-brown, more body hairs develop, and the tail becomes proportionally shorter. Mature larvae are approximately 6 mm long and 1.5 mm wide. Adult Khapra beetles have wings, but apparently do not fly and feed very little. Mated females live from four to seven days, unmated females from 20 to 30 days, and males from 7 to 12 days. Mating occurs about five days after emergence, and egg laying begins almost immediately at 40°C. Egg laying may begin at one to three days at cooler temperatures, but no eggs are produced at 20°C. Eggs hatch in 3 to 14 days after the female lays an average of 50 to 90 eggs that are loosely scattered in the host material. Complete development from egg to adult can occur within 26 to 220 days, depending upon temperature. Optimum temperature for development is 35°C. If the temperature falls below 25°C for a period of time or if larvae are very crowded, they may enter diapauses. They can survive temperatures below -8°C. In diapauses, the larvae can moult but are inactive and may remain in this condition for many years (Anonymous 1981).
 Larvae of Trogoderma granarium and damaged grains (photo: agspsrv34.agric.wa.gov.au) |
Detection/indexing methods used in the CGIAR Centres
- At ICRISAT - Dry seed examination using magnifying lens.
Treatment/control
High concentrations of fumigant (Aluminium phosphide) are maintained during the fumigation period to allow penetration into all cracks and crevices. In an eradication programme, both fumigants and surface treatment (chloropyriphos) are used in combination with preventive measures, e.g., good sanitation practices and exclusion.
Procedure followed in the CGIAR Centres in case of positive test
- At ICRISAT - Rejection and incineration of the infested seed samples.
EPPO protocols
EPPO A2 list: No.121.
Control. Methyl bromide fumigation gives good control for a wide range of commodities. Effective control in the structure of buildings and ships requires high concentrations maintained over the fumigation period to enable the gas to penetrate into cracks and crevices. A list of dosage schedules may be found in EPPO quarantine procedure No. 12 (OEPP/EPPO 1982). Phosphine can also be used against T. granarium; dosage schedules are given by OEPP/EPPO (1984). In India, the use of deoiled neem (Azadirachta indica) seed powder mixed into wheat was found to be an effective and cheap method to control the pest in stored wheat (Singh and Kataria 1986). The use of carbon dioxide was also reported to be effective in India (Srivastava 1985). As an alternative to the fumigation of cereals with methyl bromide or other pesticides, the use of a heat treatment has been reported to be very effective against the Khapra beetle (Fleurat-Lessard 1985). An exposure to 60°C for 30 min resulted in 100% mortality of all stages of T. granarium (Ismail et al. 1988).
Phytosanitary risk. T. granarium is an A2 quarantine organism for EPPO (OEPP/EPPO 1981), and is also of quarantine concern for CPPC, COSAVE, JUNAC, NAPPO and OIRSA. The continued occurrence of T. granarium on produce imported from countries where it is indigenous, and the potential for spread due to increasing use of dry cargo containers and roll-on roll-off road transport, make it a continued threat to EPPO countries. This not only applies to the risk of establishment in heated buildings in areas of unfavourable climate, but also to parts of Greece, Italy, Spain and Russia on the fringes of the natural range, where it is not known to be established. A minimum period of four months with an average temperature of 20°C is considered necessary for T. granarium to be a threat. In addition, it should be recalled that other continents take severe measures against T. granarium; the presence of the pest in an EPPO country would be a significant additional constraint to its export.
Phytosanitary measures. EPPO (OEPP/EPPO 1990) recommends that it is preferable not to require a Phytosanitary Certificate for stored products, but rather to inspect consignments on import and take appropriate post-entry action, for example treatment following EPPO Quarantine Procedures Nos 12 or 18 (OEPP/EPPO 1982; 1984).
References and further reading
Anonymous. 1981. Data sheets on quarantine organisms. Trogoderma granarium Everts. European and Mediterranean Plant Protection Organization Bulletin 11 (1) Set 4, List A2, 1-6 pp.
Fleurat-Lessard F. 1985. Les traitements thermiques de desinfestation des cereals et des produits céréaliers: possibilité d'utilisation pratique et domaine d'application. Bulletin OEPP/EPPO 15:109-118.
Ismail AY, Abid SH, Mawlood N.A. 1988. Effect of high temperature on the mortality of the red flour beetle Tribolium confusum and khapra beetle Trogoderma granarium. Zanco 1:35-42.
Lindgren DL, Vincent LE. 1959. Biology and control of Trogoderma granarium Everts. Journal of Economic Entomology 52:312-319.
Lindgren DL, Vincent LE, Krohne HE. 1955. The khapra beetle, Trogoderma granarium Everts. Hilgardia 24:1-36.
OEPP/EPPO. 1990. Specific quarantine requirements. EPPO Technical Documents No. 1008.
OEPP/EPPO. 1984. Quarantine procedures No.18. Phosphine fumigation of stored products. Bulletin OEPP/EPPO Bulletin 14:598-599.
OEPP/EPPO. 1982. Quarantine procedures No.12. Methyl bromide fumigation of stored products. Bulletin OEPP/EPPO Bulletin 12 (Special issue on EPPO recommendations on fumigation standards): 30-31.
OEPP/EPPO. 1981. Data sheets on quarantine organisms No. 121, Trogoderma granarium. Bulletin OEPP/EPPO Bulletin 11(1).
Singh RP, Kataria PK. 1986. Deoiled neem kernel powder as protectant of wheat seeds against Trogoderma granarium Everts. Indian Journal of Entomology 48:119-120.
Srivastava JL. 1985. Use of controlled atmosphere for the control of stored product insects. In: Behavioural and physiological approaches in pest management (Ed. by Regupathy, A.; Jayaraj, S.), pp. 202-207. Coimbatore, Tamil Nadu, India.
Nematodes (chickpea)
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).






