stog-forage-grass
Phytoplasma - forage grass
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
Napier Grass Stunt Disease
Scientific name
Phytoplasma belonging to 16SrXI (rice yellow dwarf) group; 16Sr111 group
Significance
Reduction in plant biomass by 20 to 40% poses a major problem to small-holder cattle producers (milk and meat). It is threatening the livestock industry and food security in east and central Africa.
Symptoms
Etiolation, reduction in leaf size, proliferation of tillers and shortened internodes
Hosts
Pennisetum purpureum, Medicago sativa, Cynodon dactylon
Geographic distribution
East and Central Africa
Biology and transmission
The method of plant propagation and the presence of insect vectors promote the spread of NGSD over long distances. Napier grass produces small, unviable seeds thus propagation is vegetative either by stem cutting or clump splitting.
Transmission of the pathogen among plants is facilitated by leafhoppers. Leafhoppers generally spend their life on one plant but can transfer to another plant during cropping or when blown by wind. Transmission of pathogen in plants happens after the latent period when phytoplasma multiplies in the body and migrates to the salivary glands of the insect. While feeding on phloem sap, the insect inoculate the pathogen into the plant.
Strains were most similar (96%) to Bermuda grass white leaf phytoplasma (Accession 16388).
Detection/indexing method in place
- at CIAT: Not applicable
- at ILRI: NASH, PCR method
Treatment/control
- If the disease is present then all symptomatic plants should be removed and burnt.
- Measures to control the spread of NGSD can be effective only if there is sufficient knowledge on vector lifecycle in relation to phytoplasma transmission.
- For high infection check for disease free seeds tillers to re-establish plots.
Procedure followed at the centers in case of positive test
- All individual plants tested, clean plants retained and infected plants rogued and material burnt
References and further reading
Arocha Y, Zerfy T, Abebe G, Proud J, Hanson J, Wilson M, Jones P, Lucas J. 2008. Identification of Potential Vectors and Alternative Plant Hosts for the Phytoplasma Associated with Napier Grass Stunt Disease in Ethiopia. Journal of Phytopathology. Published Online: 11 Nov 2008.
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Frison EA, Putter CAJ. (eds.). 1993. FAO/IBPGR Technical Guidelines for theSafe Movement of Sugarcane Germplasm. Food and Agriculture Organization of the United Nations, Rome/ International Board for Plant Genetic Resources, Rome.
Jones P, Arocha Y, Zerfy T, Proud J, Abebe G, Hanson J. 2007. A stunting syndrome of Napier grass in Ethiopia is associated with a 16SrIII Group phytoplasma, Plant Pathology, 56, 345.
Jones P, Devonshire BJ, Holman TJ, Ajanga S. 2004. Napier grass stunt: a new disease associated with a 16SrXI Group phytoplasma in Kenya. [online] Available from URL: http://www.bspp.org.uk/ndr/july2004/2004-19.asp Date accessed 13 April 2010
Mulaa M. Nov. 2004. A Survey to Collect and Identify Potential Vectors of Napier Grass stunting disease Associated with Phytoplasma in Western Kenya. Nov. 25-29, 204. ILRI, Unpublished material.
Weeds (Forage grasses)
Weeds
Viruses - forage grass
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
|
Contents: |
Scientific name
Digitaria striate mosaic virus (DiSMV or DSMV)
Significance
Minor significance for quarantine because widespread. It can affect several important forage grasses, maize and wheat.
Symptoms
Chlorotic striations and stripes
Narrow white streaks or local lesions, usually 1-3 mm long; longer wider chlorotic often yellow local lesions with diffuse margin; narrow, short, white or chlorotic streaks or local lesions with some showing diffuse edges, but most local lesions clearly defined; chlorotic streaks and local lesions, severe deformation of pant.
Hosts
Avena sativa, Brachiaria subquadripara (syn. B. miliiformis), Chloris gayana, Digitaria decumbens (D. eriantha), Digitaria ciliaris, Digitaria sanguinalis, Digitaria setigera, Dinebra retroflexa, Echinochloa colona, Eleusine indica, Hordeum vulgare, Lolium multiflorum, Sorghum bicolor, Zea mays
Geographic distribution
Worldwide
Queensland (Australia), India (Maharashtra State)
Biology and transmission
Transmitted in nature by the planthopper Sogatella kalophon but not by seeds or mechanical means.
Vetor specificity and host preference should limit the spread of DSV.
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- None, not seed borne
Procedure followed at the centers in case of positive test
- For low infection, rougue the field and burn infected plants. For high infection produce seeds in a screenhouse to obtain disease free seeds to re-establich plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Seed Health General Publication by the Center or CGIAR
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Scientific name
Elephant grass mosaic virus (EGMV)
Significance
Although EGMV has been found only in elephant grass, experimental transmission to corn and sorghum indicates that the virus maybe of economic importance.
Symptoms
Mosaic in leaves; chlorotic spots and streaks
Hosts
Andropogon schoenanthus, Avena sativa, Chenopodium amaranticolor, C. quinoa, Gomphrena, globosa, Hordeum vulgare, Oryza sativa, Panicum compressum, P. maximum, Panicum maximum, Pennisetum purpureum, Secale cereale, Stenotaphrum secundatum, Sorghum bicolor, Triticum aestivum, Zea mays
Geographic distribution
East Africa, Brazil
Biology and transmission
A virus isolated from leaves of elephant grass (Pennisetum purpureum). It was mechanically transmitted to a few cultivars of Zea mays and Sorghum bicolor, but other test plants including elephant grass could not be infected.
It was not transmitted by Myzus persicae Sulz. and Rhopalosiphum maidis Fitch.
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection check for disease free suttings to re-establish plots.
References and further reading
http://cat.inist.fr/?aModele=afficheN&cpsidt=3793303
http://www.apsnet.org/pd/PDFS/1993/PlantDisease77n07_726.PDF/
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Marins CRF, Kitajima EW. 1993. A unique virus isolated from elephant grass. Plant Disease. 77(7): 726-729.
Scientific name
Guineagrass mosaic virus (GMV)
Significance
Causes reduced yields in grasses in tropical areas and not seed borne but can affect maize.
Symptoms
Systematically infected plants show characteristic rhomboid or eye-shaped lesions on infected leaves. As the disease progresses, various mosaic patterns and chlorotic patches develop, causing early leaf senescence. As long as infected plants are maintained under favorable conditions, the disease does not cause significant damage.
Light-green or yellow mosaic
Hosts
Avena sativa, Brachiaria decumbens, B. deflexa, B. dictyoneura, B. humidicola, B. jubata, B. ruziziensis, Dactylis glomerata, Digitaria sanguinalis, Echinochloa crus-gali, Eleusine coracana, Hordeum vulgare, Panicum maximum, P. capillare, Pennisetum glaucum, Sorghum bicolor, S. sudanense, Zea mays
Geographic distribution
East Africa, West Africa, South America
Côte d’Ivoire, Brazil, Colombia
Biology and transmission
This virus is related to Johnson grass mosaic virus
Transmitted by aphids and through mechanical inoculation
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse to re-establish plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Virus of Plants. Description and List from the VIDE Database. CAB International, UK. 1484 pp.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecct. 800 pp.
Lenne JM, Trutmann P. (eds.) 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & Centro Internacional de Agricultura Tropical (CIAT), Colombia. 404 pp.
Olufemi WA, Mbiele Al, Nkouka N. (eds.) 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Seed Health General Publication Published by the Center or CGIAR
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Scientific name
Johnsongrass mosaic virus (JGMV)
Other scientific names
Maize dwarf mosaic virus — strain O (McDaniel and Gordon, 1985; Shukla et al., 1989),
Sugarcane mosaic virus — Australian Johnson grass virus (Shukla et al., 1987),
Maize dwarf mosaic virus — Kansas I strain (McKern et al., 1990).
Significance
Important disease of many forage grasses, sorghum and maize but of quarantine significance because currently not widely distributed.
Symptoms
Mosaic and variegation, ringspots and chlorosis, necrotic red stripe, necrotic red leaf; necrosis of new leaves, stunting; systemic chlorotic mosaic, mottling, necrosis, stunting
Mosaic, ring spots, stunting
Hosts
Brachiaria miliiformis, B. praetervisa, Cenchrus ciliaris, Panicum miliaceum, Paspalum orbiculare, Pennisetum typhoides, Sorghum x almum, S. bicolor, S. haplense, S. laxiflorum, S. macrospermum, S. miliaceum, S. stipoideum, S. sudanense, S. verticilliflorum, S. vulgare, Zea mays
Geographic distribution
USA, Australia, East Africa
Biology and transmission
Transmission by aphids, sap and mechanical inoculation
Vascular puncture inoculation of seedlings
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse and screen for disease free seedlings to re-establish plots
References and further reading
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Scientific name
Maize dwarf mosaic virus (MDMV)
Other scientific names
MDMV-A, MDMV-D, MDMV-E, MDMV-F; Sugarcane mosaic virus (SCMV), Sorghum red stripe virus (SRSV).
Significance
Widespread, seed and vector transmitted and also affects maize and sorghum.
Symptoms
Uneven chlorotic stripes in leaves, occasional reddening; foliar faint streak, mottle, foliar ring-like flecks; distortion and necrosis of young leaves; poor filling of cobs, stunting
Mosaic and stunting
Hosts
Avena sativa, Brachiaria eruciformis, B. platyphylla, Chloris gayana, Cynodon dactylon, Dactylis glomerata, Digitaria sanguinalis, Echinochloa crus-gali, Eleusine coracana, Eragrostis trichodes, Hordeum vulgare, Lolium perenne, Melinis minutiflora, Panicum maximum, P. sumatrense, P. capillare, Paspalum dilatatum, Poa pratensis, Rottboellia cochinchinensis, Secale cereale, Setaria viridis, Sorghum arundinaceum, S. bicolor, S. haplense, Zea mays
Geographic distribution
Australia, China, South Africa, USA
Biology and transmission
Transmitted by aphids; seed-borne
In Africa Zea mays is grown in mid to high altitudes.
Did not affect oats, rice, wheat, soybeans and cowpeas.
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA.
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse and screen for disease free seedlings to re-establish plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Virus of Plants. Description and List from the VIDE Database. CAB International, UK. 1484 pp.
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecct. 800 pp.
Lenne JM, Trutmann P. (eds.) 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & Centro Internacional de Agricultura Tropical (CIAT), Colombia. 404 pp.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Olufemi WA, Mbiele AL, Nkouka N. (eds.) 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Scientific name
Maize streak virus (MSV)
Other scientific names
Bajra streak virus (Seth et al., 1972a & 1972b), Cereal African streak virus, Maize streak A virus
Significance
Causes severe streaking and yield loss in maize and other grasses.
Quarantine significance because not found in the Americas.
Symptoms
White chlorotic spots along veins of unfolding leaf; chlorotic streaking to uniform chlorosis; necrosis, local lesions, stunting; chlorotic or white streaking or lesions; systemic chlorotic streaking
Chlorotic streaking and various other foliar lesions.
Hosts
Agropyron cristatum, A. fragile, Agrostis gigantean, Andropogon gerardii, Arrhenatherum elatius, Avena sativa, Axonopus compressus, Bothriochloa barbinodis, Brachiara deflexa, B. lata, B. reptans, B.villosa (B. distichophylla), Bromus erectus, B. inermis, B. catharticus, Cenchrus ciliaris, Chloris gayana, Coix lacryma-jobi, Cymbopogon schoenanthus, Cynodon dactylon, Dactylis glomerata, Dactyloctenium gianteum, Digitaria abyssinica, Digitaria velutina, D. milanjiana, D. ternata, D. eriantha, D. horizontalis, D. sanguinalis, Echinochloa colona, E. crus-gali, E. polystachya, Eleusine coracana, E. indica, Eragrostis curvula, Fesuca ovina, F. pratensis, F. rubra, Heteropogon contortus, Holcus lanatus, Hordeum vulgare, Hyparrhenia rufa, Leersia hexandra, Lolium multiflorum, L. perenne, L. rigidum, Panicum coloratum, P. maximum, P. bergii, P. sumatrense, Paspalum dilatatum, P. notatum, P. scorbiculatum, P. almum, P. urvillei, Pennisetum clandestinum, P. purpureum, P. glaucum, Phalaris aquatica, P. arundinacea, Phleum pretense, Poa pretense, Rottboellia cochinchinensis, Setaria sphacelata, S. pumila, S. verticillata, S. megaphylla, S. homonyma, S. viridis, Sorghum arundinaceum, S. bicolor, Tripsacum dactyloides, Urochloa panicoides, U. trichopus, Zea mays
Geographic distribution
Madagascar, East Africa, Yemen, Reunion, India
Biology and transmission
This virus is transmitted by insects belonging to Cicadelllidae (Cicadulina mbila, Cicadulina triangular, Cicadulina zeae, Cicadulina storeiy) in a persistent manner; transmissible to seedlings by vascular puncture inoculation (VPI), but not through seed or by mechanical means; non-sap transmissible.
Detection/indexing method
- at CIAT: not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- Chemical control of the leafhopper vector is only justified for special purposes such as germplasm or seed muliplication.
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse and screen for disease free seedlings to re-establish plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Virus of Plants. Description and List from the VIDE Database. CAB International, UK. 1484 pp.
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecct. 800 pp.
Lenne JM, Trutmann P. (eds.) 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & Centro Internacional de Agricultura Tropical (CIAT), Colombia. 404 pp.
Miles JW, Maass Bl, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Olufemi WA, Mbiele AL, Nkouka N. (eds.) 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Scientific name
Sugarcane mosaic virus (SCMV)
Other scientific names
Grass mosaic virus, Maize dwarf mosaic virus MDMV-B MDMV-A, Sorghum red stripe virus (SRSV)
Significance
Worldwide spread and yield loss in some forages.
Symptoms
Foliar mottling; general mosaic to oblong chlorotic spots; oblong necrotic spots dispersed in leaves; necrotic local lesions, then systemic mosaic, necrosis
Mosaic in different variegated patterns, depending on the age of the plant and time of inoculation.
Hosts
Brachiaria eruciformis
Brachiaria spp.
Geographic distribution
Worldwide
Australia, East Africa
Biology and transmission
Transmitted by aphids; spread through infected seeds, sap, vegetative propagules and by mechanical means
Transmissible to seedlings by vascular puncture inoculation (VPI)
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: NASH, PCR method
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse and screen for disease free seedlings to re-establish plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Virus of Plants. Description and List from the VIDE Database. CAB International, UK. 1484 pp.
Frison EA, Putter CAJ. (eds.). 1993. FAO/IBPGR Technical Guidelines for theSafe Movement of Sugarcane Germplasm. Food and Agriculture Organization of the United Nations, Rome/ International Board for Plant Genetic Resources, Rome.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecct. 800 pp.
Lenne JM, Trutmann P. (eds.) 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & Centro Internacional de Agricultura Tropical (CIAT), Colombia. 404 pp.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Olufemi WA, Mbiele AL, Nkouka N. (eds.) 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Sukumar C, Leath KT, Skipp RS, Pederson GA, Bray RA, Latch GCM, Jr Nutter FW. (eds.) Pasture and Forage Crop Pathology. American Society of Agronomy, Inc. Crop Science Society of America, Inc., Soil Science Society of America, Inc. 653 pp.
Insects - forage grass
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
|
Contents: |
Scientific names
Acantoscelides sp, A. obtectus, A. obvelatus, A. argillaceus
Significance
No precise information on losses in stored beans by bruchids is available. However, farm storage for six month is accompanied by about 40% loss in weight with as much as 80 % of the sees bein infested and unfit for human consumption. Losses vary between 7 % in Colombia to 73 % in Kenia.
The Bruchidae are adapted to attack the mature seeds of legumes, and those which attack food legumes demonstrate a certain degree of specialization to different legume species. The larva penetrates the seed and subsequent development. Takes place entirely within the seed cotyledons. The larva pupates within the seed but before doing so prepares the point of its eventual escape from the seed by chewing away a circular escape tunnel through the cotyledon leaving intact only the testa at the outside. This area of undermined testa can be seen clearly as a grey ‘window’.
Hosts
Is a widely distributed pest of storage beans.
Geographic distribution
Cosmpolitan.
Biology and transmission
Bean weevils are generally compact and oval in shape, with small heads somewhat bent under. Sizes range to 1 mm, up to 22 mm for some tropical species. Colors are usually black or brown often with mottled patterns. Although their mandible may be elongate they do not have long snouts. Adults deposit eggs on seeds, then the larvae chew their way into the seed. When ready to pupate, the larvae typically cut an exit hole, and then return to their feeding chamber. Adult weevils have a habit of feigning death and dropping from a plant when disturbed. Typically infest various kinds of beans, living for most of their lives inside a single seed. Acantoscelides obtectus and Acantascelides obvelatus – develop in Phaseolus vulgaris; Acantascelides argillaceus – develop in Phaseolus lunatus. A. obtectus predominates in cooler areas. Eggs are being laid on stored beans or in cracks of growing pods in the field. The larvae tunnel into the seeds to feed. Adult weevils are short-lived and do little feeding. A. obtectus is a better competitor than Zabrotes subfasciatus at lower temperatures and will eventually predominate under these conditions. A. obtectus females do not glue eggs to the test but scatter them among stored seeds or infest beans in the field by ovipositing on growing pods. The newly hatched larvae will later penetrate the seed. A. obtectus live 14 days and lay an average of 45 eggs.
Detection/indexing method in place at the CGIAR Center at CIAT
- Direct visual inspection
Treatment/control
- The actual chemical control of harmful species from Coleoprera Order is acceptable only in some and verylimited situations, and inacceptable in the majority of cases for the residual toxicity The following controls are recommended: Preventive measures of control: building repairs, overall cleaning measures, specific preventive measures, physical treatments; Preventive measures applied when introducing the products into the storehouse; Periodical supervision measures Curative, non-polluting measures (dehydration powders, means of entheroleter, means of pheromone traps, use of microwave electromagnetic generators, use of strong electric fields and “corona” discharges in alternative current, use of vegetal insecticides.
Procedure followed at the centers in case of positive test
- Not noted
References and further reading
Porca M, Ghizdavu I, Oltean I, Bunescu H. 2003. Control of the coleopteres in stored agricultural products bynot-chemical methods. Journal of Central European Agriculture (online), 218 Volume 4 (2003) No3.
Seed Health General Publication Published by the Center or CGIAR
 Acanthoscelides obtectus (photos: Beans Entomology, CIAT and Miroslav Deml, Biolib) |
Scientific names
Zabrotes spp. Zabrotes subfasciatus (Boheman)
Other names
Zabrotes pectoralis (Sharp), Spermophagus subfasciatus Boheman, Spermophagus musculus Boheman, Spermophagus pectoralis Sharp, Spermophagus semifasciatus Boheman.
Significance
It is the most important pest of storage beans in warmer regions.
Symptoms
Not noted
Hosts
Beans, vigna unguiculata (cowpea). All the known hosts of Zabrotes are in the Fabaceae with a questionable record in the Bixaceae.
Geographic distribution
Cosmopolitan. Ii is a tropical species and is found predominantly in warmer areas.
Biology and transmission
Zabrotes subfasciatus attaches the egg to the seed. After hatching, the young larvae bore through their egg shell and the seed coat in one process. Zabrotes subfasciatus does not attack in the field. Adults exhibit strong sexual dimorphism. Females are large and have fourcharacteristic crfeam-colored spots on the elytra. The male is entirely brown. At 28 oC and 75%-80% r.h., females lay an average of 36 eggs and live 13 days. The egg stage lasts five to six days, larval development takes 14 days, and the pupal stage takes six to seven days.
Detection/indexing method in place at the CGIAR Center at CIAT
- Direct visual inspection
Treatment/control
- The actual chemical control of harmful species from Coleoprera Order is acceptable only in some and verylimited situations, and inacceptable in the majority of cases for the residual toxicity The following controls are recommended: Preventive measures of control: building repairs, overall cleaning measures, specific preventive measures, physical treatments; Preventive measures applied when introducing the products into the storehouse; Periodical supervision measures Curative, non-polluting measures (dehydration powders, means of entheroleter, means of pheromone traps, use of microwave electromagnetic generators, use of strong electric fields and “corona” discharges in alternative current, use of vegetal insecticides.
References of protocols at EPPO, NAPPO or other similar organization
Porca M*, Ghizdavu I, Oltean I, Bunescu H. 2003. Control of the coleopteres in stored agricultural products bynot-chemical methods. Journal of Central European Agriculture (online), 218 Volume 4 (2003) No3.
 Zabrotes subfasciatus (photos: CIAT and http://entmuseum9.ucr.edu/) |
Best practices for safe transfer of forage grass germplasm
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
|
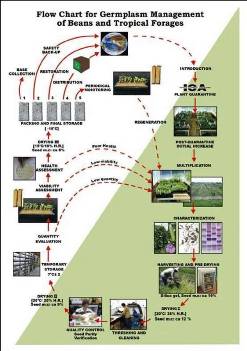
Flowchart 1.Germplasm management of Beans and Tropical Forages (Click to increase the size) |
The agreement between the CIAT and the International Treaty on Plant Genetic Resources for Food and Agriculture implies of the Genetic Resources Program (GRP) the conservation on behalf of the countries of 65,505 materials for 745 plant species of bean, cassava and tropical pastures as the biological patrimony of 141 countries. This responsibility of conservation is associated with the distribution of samples of this patrimony (517,916 samples distributed to 136 countries to the date) according to a regulation defined by the countries inside the Agreement, and phytosanitary procedure established by the Colombian Agricultural Institute.
The job of the GRP is to safeguard the genetic diversity of beans, cassava, forages, and their wild relatives through a mix of conservation methods, both in situ (in a natural outdoor habitat) and ex situ (in the controlled environment of a gene bank). Among the GRP's activities are research to improve conservation methods (including ways to minimize risks to the collections); screening germplasm for diseases and certifying it; duplicating materials of the collection; collecting or otherwise acquiring novel materials; recording passport, characterization and evaluation data for accessions of the collections (See Flowchart 1).
Phytosanitary risks are associated with international movement of germplasm, especially the inadvertent transport of pathogens and pests of quarantine significance. To minimize the phytosanitary risks associated with exchange germplasm and to ensure that it is free from pests and pathogens of quarantine significance; CIAT has implemented regulatory measures and safeguards to complement quarantine guidelines. The process, wich is supervised by the the Agricultural Colombian Institute (ICA), includes the following activities:
- Minimize the risk of accidental introduction of exotic pest and pathogens into Colombia;
- Inspect plant and facilities in screenhouses and grenhouses where the imported germplasm is being incresased;
- Inspect plants in fields and greenhouses where the germplasm intended for international export is produced; and
- Determine the seed health status of germplasm for international export.
The responsibility over the areas dealing with animal and plant health, with regard to international trade, has been bestowed upon the Agricultural Colombian Institute, within regulatory decree 1840 of 1994 “for which (ICA) has the mission of preventing the risk of the entry, spread, and establishment of exotic diseases, those of national sanitary concern, and of chemical risks, and protecting the sanitary quality of animals, plants and products that are exported, to minimize losses in animal-plant production and contribute to the security in foodstuffs”.
ICA has established quarantine procedures to regulate the introduction of plant germplasm and the issuing of phytosanitary certificates that accompany exports. ICA has a Plant Quarantine Station at an altitude of 2600 m (4o 42’ N latitude and 74o 12’W longitude) near Mosquera (Cundinamarca), about 15 km west of Santafé de Bogotá. ICA provides the regulatory mechanism for germplasm exchange following guidelines of the International Plant Protection Convention (IPPC). The recommendations of the IPPC were adopted by Colombian Congress in 1981 and implemented under decrees 501 of 1989.
In order to facilitate the importation and exportation, the ICA has developed the SISPAP, which may accessed through the ICA webpage in which the parties interested in importing and exporting plant products may become aware of the following via internet.
In Colombia, additional guidelines to reduce phytosanitary risks are in place. These safeguards are implemented according to the geographic origin and economic importance of the plant species concerned, and characteristic of potential pathogens and pests. Currently, ICA has a plant quarantine agreement with CIAT establishing which guidelines and safeguards are updated to facilitate germplasm exchange according to national and international requirements. An agreement was signed in 1981 between CIAT and ICA for quarantine procedures to regulate the introductions of germplasm into Colombia. The agreement permits the transit of seed through customs and quarantine stations according to the level of potential risk of introducing pests and diseases no yet reported in Colombia. High risk areas include Africa, Asia, and some European countries. The agreement covers not only crops for wich CIAT has a mandate but also other crops of economic importance to Colombia.
After clearance, materials pass through a step of multiplication in greenhouse stage, and the harvested seeds are subsequently planted in isolated fields. During these two steps, a phytosanitary follow-up is carried out. Plants showing any symptom of fungal, bacterial or viral disease are destroyed.
CIAT facilities for germplasm health testing are designed as a multifunctional laboratory to test seeds and tissues for fungi, bacteria, viruses, and occasionally nematodes and insects (See table below). The purpose of the Germplasm Health Laboratory (GHL) is to ensure that the designated germplasm is kept under the international phytosanitary standards for each crop commodity, and also to ensure that the germplasm distributed by GRP is free of diseases of quarantine importance (listed in the table below).
The ICA Plant Quarantine Officer, stationed at CIAT, carries out field and greenhouses inspections and issues "ICA Phytosanitary Certificate" bases upon those inspections and results obtained by GHL. This document accompanies all out-going germplasm from Colombia (“decrees 1840 of 1994 articule 3”).
The germplasm leaving CIAT and the one used for exchange, conservation, and characterization in the GRP are multiplied in isolated fields under favorable ecological conditions with supervision by ICA quarantine officers. The GRP has seed multiplication sites in Popayan (Cauca) and Santander de Quilichao (Cauca). The harvested seeds are analyzed by GHL prior to shipment abroad or long-term conservation.
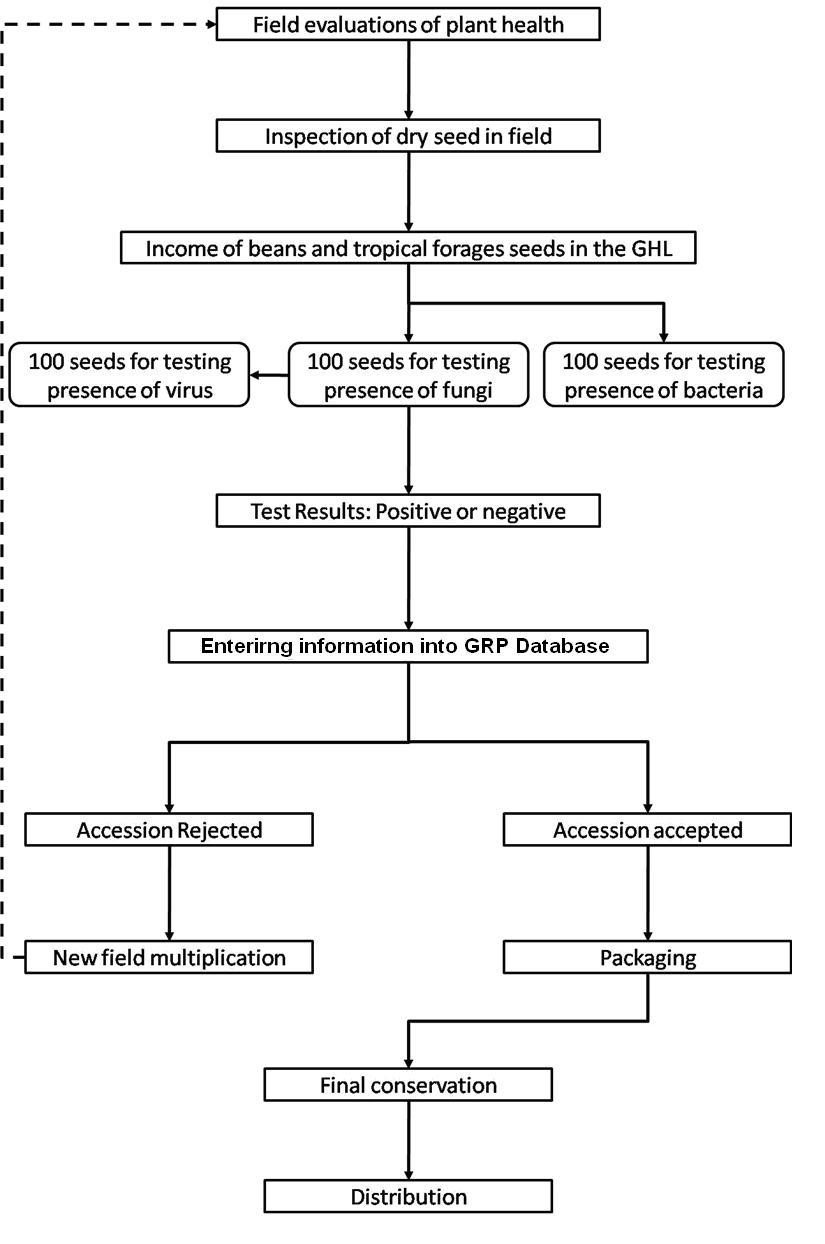
Seed health testing activities include:
- Reception, registration, sampling and storage of incoming material.
- Preparation of working samples for testing.
- Analysis.
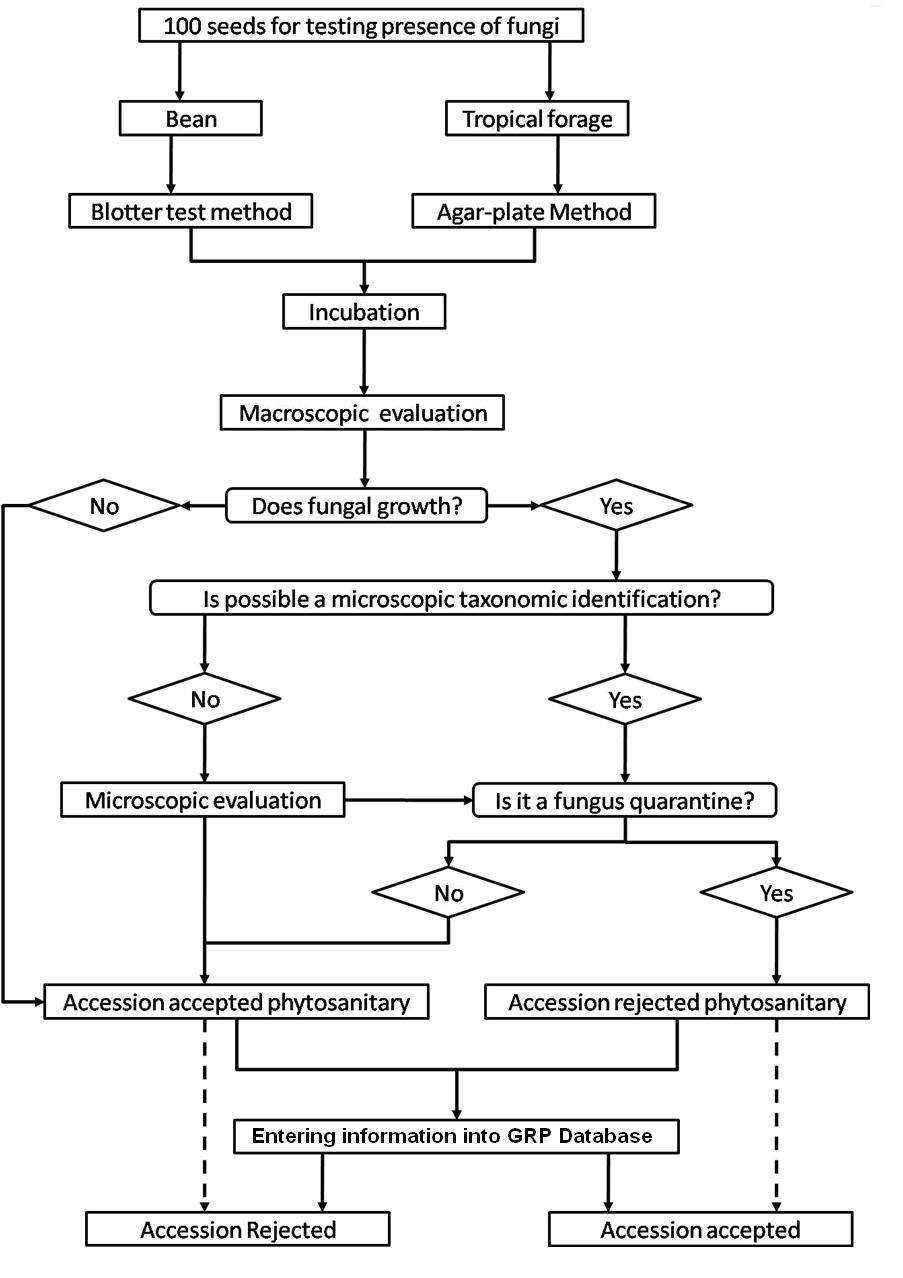
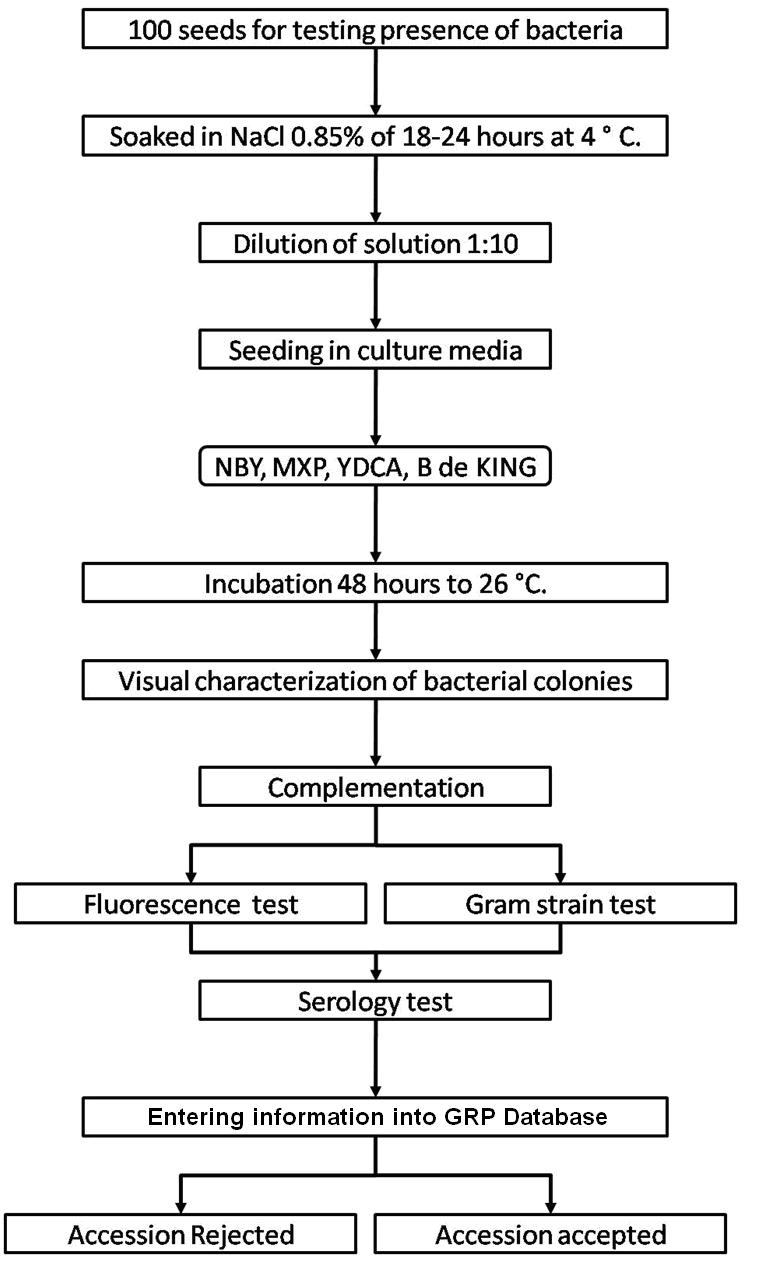
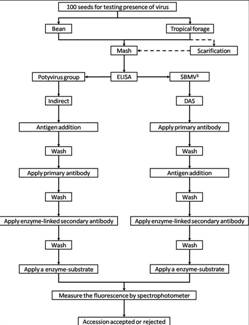
The seed health testing methods used at CIAT for beans and tropical pastures are summarized in The Handbook of Procedures of the Germplasm Health Laboratory (see also Flows charts 2, 3, 4, 5 below, for a quick reference).
To detect pathogens of quarantine significance, the GHL uses the methodologies recommended by CIAT pathologists and virologists (see table below). When a recipient country has additional requirements, the GHL carries out additional tests wherever possible to comply with the specific quarantine regulations of the recipient country. The GHL realizes additional researches in the management and characterization of pathogens of quarantine importance and in the standardization of new methodologies of diagnosis that are more effective and sensitive.
ILRI carries out pathogen detection tests before each regeneration. Seeds are withdrawn from the genebank, scarified and germinated using appropriate conditions for the species. Seedlings are transferred to sterilized compost in seedling trays after germination and grown in a virus screened area until one to three leaf stage. Visual inspection is carried out and seedlings are batch tested in batches of 10 seedlings using TBIA for common grass viruses. Individual seedling tests are performed on any positive batches for confirmation. Clean seedlings are released to the field for regeneration.
If the seedlings are infected with seed borne pathogens and more original seeds are available for a second regeneration, the seedlings are destroyed by incineration. If no original seeds are available, the seedlings are transferred to large pots and retained in a virus screened area for seed production. Since virus transmission is rarely 100%, the seeds are harvested from infected plants and germinated. The seedlings are tested as above to identify clean seedlings for release to the field. If all seeds are infected, thermotherapy and meristem culture are used to eliminate the virus and obtain clean plantlets in in vitro culture. Rooted plantlets can be acclimatized in the greenhouse and these plants used as a source of clean seeds.
Plants in the field are regularly inspected for virus symptoms and samples taken for testing with either TBIA or ELISA for common grass viruses. Some grass species are maintained in the field genebank and also require regular inspection. Young leaves are sampled from Napier grass for diagnosis for phytoplasma associated with Napier grass stunt using NASH. Plants that test positive for virus diseases are rogued from the field.
Field inspection is also carried out for pathogenic fungi and insects and any infection is controlled through field management. Regeneration and field genebank plots are sprayed with fungicides or insecticides at early signs of infection. Regular cutting is done on grass plants in the field genebank to reduce fungal diseases.
ILRI uses a range of different methods to detect pathogens of forage grass germplasm. Click the forage grasses health table for specific species information about health diagnosis methods to detect some of these diseases. Detailed protocols were developed for the detection of:
- Virus (ELISA and TBIA).
- Phytoplasm (NASH).
- Fungi (seed washing technique; blotter test; potato dextrose agar; oatmeal agar).
References and further reading
Cuervo MI, Balcazar MS, Ramirez JL, Medina CA, Debouck D. 2009. Manual de operaciones laboratorio sanidad de germoplasma – unidad de recursos genéticos. GRP, CIAT, Colômbia. 72 pp. Available here
Plant Biosecurity, Biosecurity Australia 2000. Viruses, Phytoplasmas and Spiroplasmas of Clonal Grasses and Their Diagnosis. Consultancy report. Available from: http://www.daff.gov.au/__data/assets/word_doc/0020/24761/consul_rpt_clonal.doc. Date accessed: 30 March 2010.