stog-forage-legume
Weeds (Forage legumes)
Weeds
Insects - forage legume
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell); ICARDA, Syria (Siham Asaad).
|
Contents: |
Scientific names
Acantoscelides sp, A. obtectus, A. obvelatus, A. argillaceus
Significance
No precise information on losses in stored beans by bruchids is available. However, farm storage for six month is accompanied by about 40% loss in weight with as much as 80 % of the sees bein infested and unfit for human consumption. Losses vary between 7 % in Colombia to 73 % in Kenia.
The Bruchidae are adapted to attack the mature seeds of legumes, and those which attack food legumes demonstrate a certain degree of specialization to different legume species. The larva penetrates the seed and subsequent development. Takes place entirely within the seed cotyledons. The larva pupates within the seed but before doing so prepares the point of its eventual escape from the seed by chewing away a circular escape tunnel through the cotyledon leaving intact only the testa at the outside. This area of undermined testa can be seen clearly as a grey ‘window’.
Hosts
Is a widely distributed pest of storage beans.
Geographic distribution
Cosmpolitan.
Biology and transmission
Bean weevils are generally compact and oval in shape, with small heads somewhat bent under. Sizes range to 1 mm, up to 22 mm for some tropical species. Colors are usually black or brown often with mottled patterns. Although their mandible may be elongate they do not have long snouts. Adults deposit eggs on seeds, then the larvae chew their way into the seed. When ready to pupate, the larvae typically cut an exit hole, and then return to their feeding chamber. Adult weevils have a habit of feigning death and dropping from a plant when disturbed. Typically infest various kinds of beans, living for most of their lives inside a single seed. Acantoscelides obtectus and Acantascelides obvelatus – develop in Phaseolus vulgaris; Acantascelides argillaceus – develop in Phaseolus lunatus. A. obtectus predominates in cooler areas. Eggs are being laid on stored beans or in cracks of growing pods in the field. The larvae tunnel into the seeds to feed. Adult weevils are short-lived and do little feeding. A. obtectus is a better competitor than Zabrotes subfasciatus at lower temperatures and will eventually predominate under these conditions. A. obtectus females do not glue eggs to the test but scatter them among stored seeds or infest beans in the field by ovipositing on growing pods. The newly hatched larvae will later penetrate the seed. A. obtectus live 14 days and lay an average of 45 eggs.
Detection/indexing method in place at the CGIAR Center at CIAT
- Direct visual inspection
Treatment/control
- The actual chemical control of harmful species from Coleoprera Order is acceptable only in some and verylimited situations, and inacceptable in the majority of cases for the residual toxicity The following controls are recommended: Preventive measures of control: building repairs, overall cleaning measures, specific preventive measures, physical treatments; Preventive measures applied when introducing the products into the storehouse; Periodical supervision measures Curative, non-polluting measures (dehydration powders, means of entheroleter, means of pheromone traps, use of microwave electromagnetic generators, use of strong electric fields and “corona” discharges in alternative current, use of vegetal insecticides.
Procedure followed at the centers in case of positive test
- Not noted
References and further reading
Porca M, Ghizdavu I, Oltean I, Bunescu H. 2003. Control of the coleopteres in stored agricultural products bynot-chemical methods. Journal of Central European Agriculture (online), 218 Volume 4 (2003) No3.
Seed Health General Publication Published by the Center or CGIAR
 Acanthoscelides obtectus(photos: Beans Entomology, CIAT and Miroslav Deml, Biolib) |
Scientific names
Zabrotes spp. Zabrotes subfasciatus (Boheman)
Other names
Zabrotes pectoralis (Sharp), Spermophagus subfasciatus Boheman, Spermophagus musculus Boheman, Spermophagus pectoralis Sharp, Spermophagus semifasciatus Boheman.
Significance
It is the most important pest of storage beans in warmer regions.
Symptoms
Not noted
Hosts
Beans, vigna unguiculata (cowpea). All the known hosts of Zabrotes are in the Fabaceae with a questionable record in the Bixaceae.
Geographic distribution
Cosmopolitan. Ii is a tropical species and is found predominantly in warmer areas.
Biology and transmission
Zabrotes subfasciatus attaches the egg to the seed. After hatching, the young larvae bore through their egg shell and the seed coat in one process. Zabrotes subfasciatus does not attack in the field. Adults exhibit strong sexual dimorphism. Females are large and have fourcharacteristic crfeam-colored spots on the elytra. The male is entirely brown. At 28 oC and 75%-80% r.h., females lay an average of 36 eggs and live 13 days. The egg stage lasts five to six days, larval development takes 14 days, and the pupal stage takes six to seven days.
Detection/indexing method in place at the CGIAR Center at CIAT
- Direct visual inspection
Treatment/control
- The actual chemical control of harmful species from Coleoprera Order is acceptable only in some and verylimited situations, and inacceptable in the majority of cases for the residual toxicity The following controls are recommended: Preventive measures of control: building repairs, overall cleaning measures, specific preventive measures, physical treatments; Preventive measures applied when introducing the products into the storehouse; Periodical supervision measures Curative, non-polluting measures (dehydration powders, means of entheroleter, means of pheromone traps, use of microwave electromagnetic generators, use of strong electric fields and “corona” discharges in alternative current, use of vegetal insecticides.
References of protocols at EPPO, NAPPO or other similar organization
Porca M*, Ghizdavu I, Oltean I, Bunescu H. 2003. Control of the coleopteres in stored agricultural products bynot-chemical methods. Journal of Central European Agriculture (online), 218 Volume 4 (2003) No3.
 Zabrotes subfasciatus(photos: CIAT and http://entmuseum9.ucr.edu/) |
Best practices for the safe transfer of forage legume germplasm
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell); ICARDA, Syria (Siham Asaad).
|
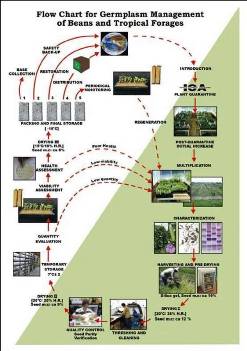
Flowchart 1.Germplasm management of Beans and Tropical Forages (Click to increase the size) |
The agreement between the CIAT and the International Treaty on Plant Genetic Resources for Food and Agriculture implies of the Genetic Resources Program (GRP) the conservation on behalf of the countries of 65,505 materials for 745 plant species of bean, cassava and tropical pastures as the biological patrimony of 141 countries. This responsibility of conservation is associated with the distribution of samples of this patrimony (517,916 samples distributed to 136 countries to the date) according to a regulation defined by the countries inside the Agreement, and phytosanitary procedure established by the Colombian Agricultural Institute.
The job of the GRP is to safeguard the genetic diversity of beans, cassava, forages, and their wild relatives through a mix of conservation methods, both in situ (in a natural outdoor habitat) and ex situ (in the controlled environment of a gene bank). Among the GRP's activities are research to improve conservation methods (including ways to minimize risks to the collections); screening germplasm for diseases and certifying it; duplicating materials of the collection; collecting or otherwise acquiring novel materials; recording passport, characterization and evaluation data for accessions of the collections (See Flowchart 1).
Phytosanitary risks are associated with international movement of germplasm, especially the inadvertent transport of pathogens and pests of quarantine significance. To minimize the phytosanitary risks associated with exchange germplasm and to ensure that it is free from pests and pathogens of quarantine significance; CIAT has implemented regulatory measures and safeguards to complement quarantine guidelines. The process, wich is supervised by the the Agricultural Colombian Institute (ICA), includes the following activities:
- Minimize the risk of accidental introduction of exotic pest and pathogens into Colombia;
- Inspect plant and facilities in screenhouses and grenhouses where the imported germplasm is being incresased;
- Inspect plants in fields and greenhouses where the germplasm intended for international export is produced; and
- Determine the seed health status of germplasm for international export.
The responsibility over the areas dealing with animal and plant health, with regard to international trade, has been bestowed upon the Agricultural Colombian Institute, within regulatory decree 1840 of 1994 “for which (ICA) has the mission of preventing the risk of the entry, spread, and establishment of exotic diseases, those of national sanitary concern, and of chemical risks, and protecting the sanitary quality of animals, plants and products that are exported, to minimize losses in animal-plant production and contribute to the security in foodstuffs”.
ICA has established quarantine procedures to regulate the introduction of plant germplasm and the issuing of phytosanitary certificates that accompany exports. ICA has a Plant Quarantine Station at an altitude of 2600 m (4o 42’ N latitude and 74o 12’W longitude) near Mosquera (Cundinamarca), about 15 km west of Santafé de Bogotá. ICA provides the regulatory mechanism for germplasm exchange following guidelines of the International Plant Protection Convention (IPPC). The recommendations of the IPPC were adopted by Colombian Congress in 1981 and implemented under decrees 501 of 1989.
In order to facilitate the importation and exportation, the ICA has developed the SISPAP, which may accessed through the ICA webpage in which the parties interested in importing and exporting plant products may become aware of the following via internet.
In Colombia, additional guidelines to reduce phytosanitary risks are in place. These safeguards are implemented according to the geographic origin and economic importance of the plant species concerned, and characteristic of potential pathogens and pests. Currently, ICA has a plant quarantine agreement with CIAT establishing which guidelines and safeguards are updated to facilitate germplasm exchange according to national and international requirements. An agreement was signed in 1981 between CIAT and ICA for quarantine procedures to regulate the introductions of germplasm into Colombia. The agreement permits the transit of seed through customs and quarantine stations according to the level of potential risk of introducing pests and diseases no yet reported in Colombia. High risk areas include Africa, Asia, and some European countries. The agreement covers not only crops for wich CIAT has a mandate but also other crops of economic importance to Colombia.
After clearance, materials pass through a step of multiplication in greenhouse stage, and the harvested seeds are subsequently planted in isolated fields. During these two steps, a phytosanitary follow-up is carried out. Plants showing any symptom of fungal, bacterial or viral disease are destroyed.
CIAT facilities for germplasm health testing are designed as a multifunctional laboratory to test seeds and tissues for fungi, bacteria, viruses, and occasionally nematodes and insects (See table below). The purpose of the Germplasm Health Laboratory (GHL) is to ensure that the designated germplasm is kept under the international phytosanitary standards for each crop commodity, and also to ensure that the germplasm distributed by GRP is free of diseases of quarantine importance (listed in the table below).
The ICA Plant Quarantine Officer, stationed at CIAT, carries out field and greenhouses inspections and issues "ICA Phytosanitary Certificate" bases upon those inspections and results obtained by GHL. This document accompanies all out-going germplasm from Colombia (“decrees 1840 of 1994 articule 3”).
The germplasm leaving CIAT and the one used for exchange, conservation, and characterization in the GRP are multiplied in isolated fields under favorable ecological conditions with supervision by ICA quarantine officers. The GRP has seed multiplication sites in Popayan (Cauca) and Santander de Quilichao (Cauca). The harvested seeds are analyzed by GHL prior to shipment abroad or long-term conservation.
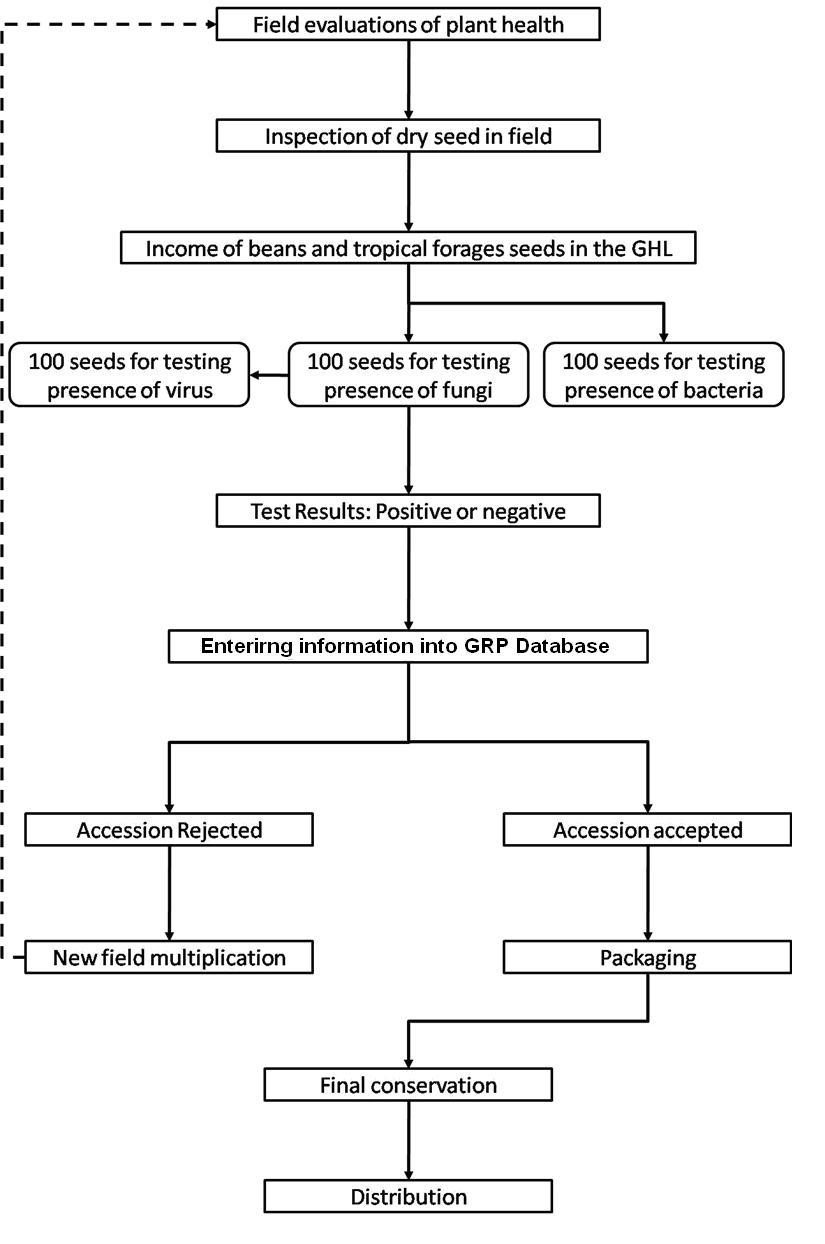
Seed health testing activities include:
- Reception, registration, sampling and storage of incoming material.
- Preparation of working samples for testing.
- Analysis.
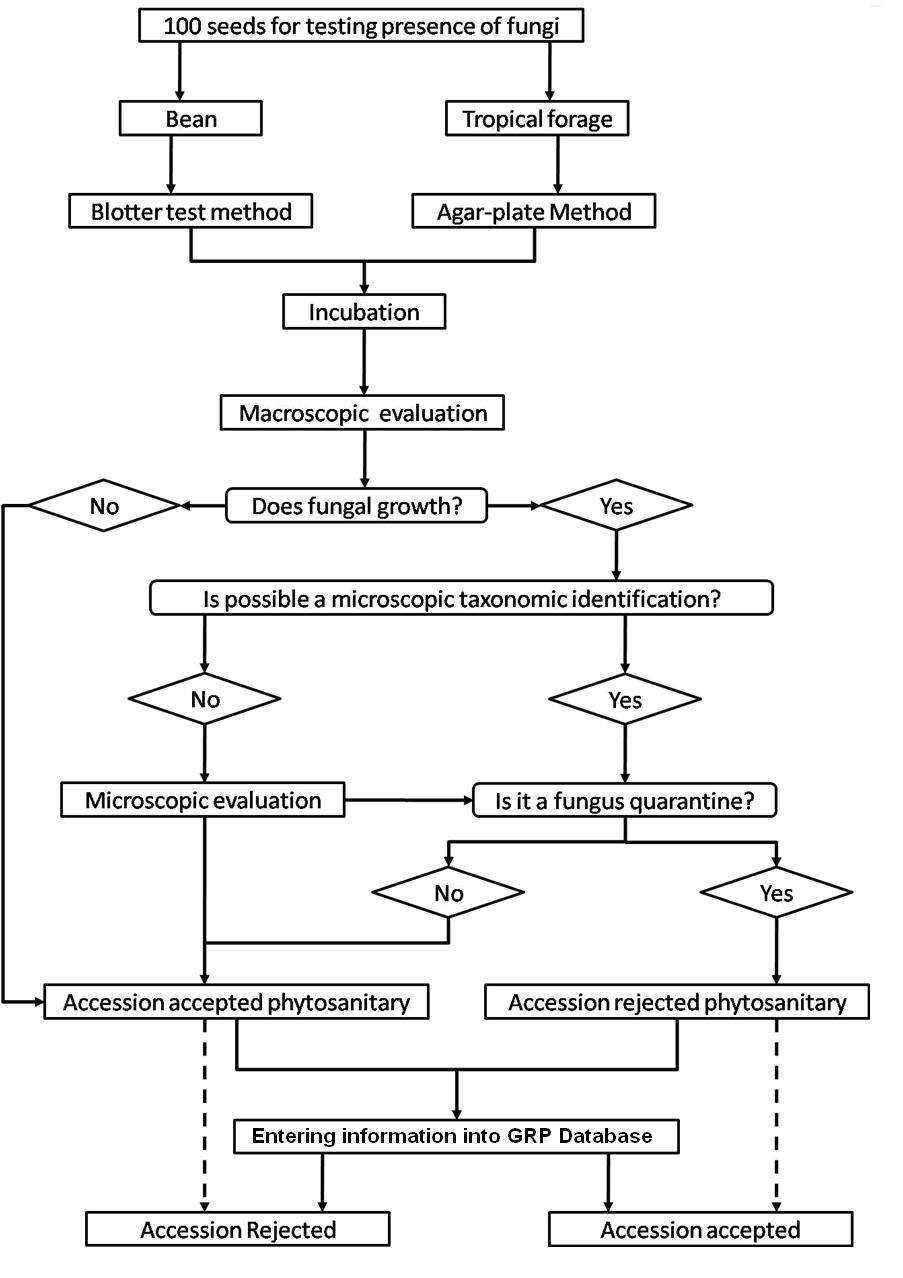
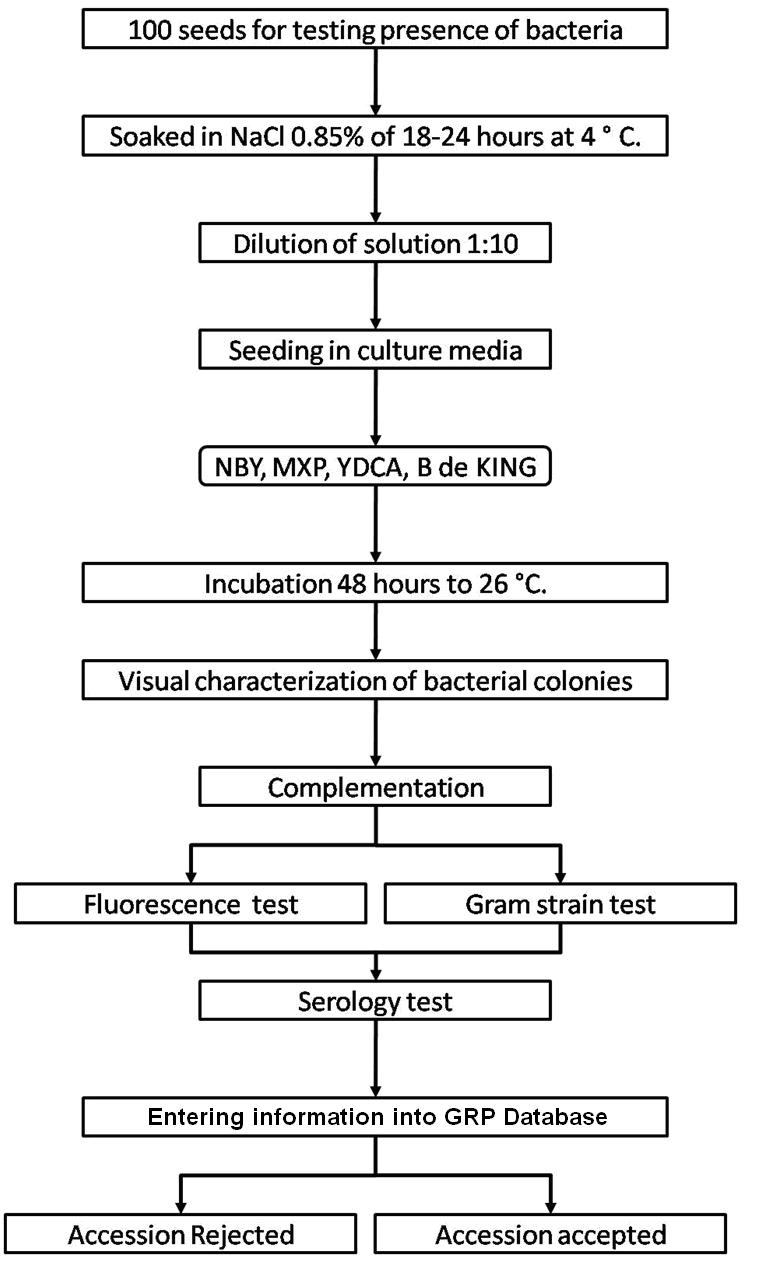
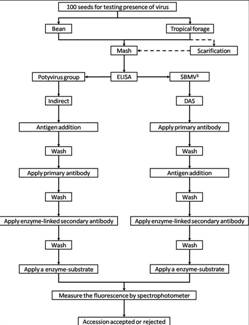
The seed health testing methods used at CIAT for beans and tropical pastures are summarized in The Handbook of Procedures of the Germplasm Health Laboratory (see also Flows charts 2, 3, 4, 5 below, for a quick reference).
To detect pathogens of quarantine significance, the GHL uses the methodologies recommended by CIAT pathologists and virologists (see table below). When a recipient country has additional requirements, the GHL carries out additional tests wherever possible to comply with the specific quarantine regulations of the recipient country. The GHL realizes additional researches in the management and characterization of pathogens of quarantine importance and in the standardization of new methodologies of diagnosis that are more effective and sensitive.
ILRI carries out virus detection tests before each regeneration. Seeds are withdrawn from the genebank, scarified and germinated using appropriate conditions for the species. Seedlings are transferred to sterilized compost in seedling trays when the radicle is from 1-5mm long and grown in a virus screened area until one to three leaf stage. Visual inspection is carried out and seedlings are batch tested in batches of 10 seedlings using TBIA for common legume viruses. Individual seedling tests are performed on any positive batches for confirmation. Clean seedlings are released to the field for regeneration.
If the seedlings are infected with seed borne pathogens and more original seeds are available for a second regeneration, the seedlings are destroyed by incineration. If no original seeds are available, the seedlings are transferred to large pots and retained in a virus screened area for seed production. Since virus transmission is rarely 100%, the seeds are harvested from infected plants and germinated. The seedlings are tested as above to identify clean seedlings for release to the field. If all seeds are infected thermotherapy and meristem culture are used to eliminate the virus and obtain clean plantlets in in vitro culture. Rooted plantlets can be acclimatized in the greenhouse and these plants used as a source of clean seeds.
Many forage legumes are perennial and can be infected in the field or are direct sown and should be tested during the growing season. Plants in the field are regularly inspected for virus symptoms and samples taken for testing with either TBIA or ELISA for common legume viruses. Plants that test positive for seed borne virus diseases are rogued from the field.
Field inspection is also carried out for pathogenic fungi and insects and any infection is controlled through field management. Regeneration and field genebank plots are sprayed with fungicides or insecticides at early signs of infection.
ILRI uses a range of different methods to detect pathogens of forage legume germplasm. Click the forage legume health table for specific species information about health diagnosis methods to detect some of these diseases. Detailed protocols were developed for the detection of:
- Virus (ELISA and TBIA).
- Fungi (seed washing technique; blotter test; potato dextrose agar; oatmeal agar).
References and further reading
Cuervo MI, Balcazar MS, Ramirez JL, Medina CA, Debouck D. 2009. Manual de operaciones laboratorio sanidad de germoplasma – unidad de recursos genéticos. GRP, CIAT, Colômbia. 72 pp. Click here to open publication
Viruses - forage legume
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell); ICARDA, Syria (Siham Asaad).
Alfalfa mosaic virus (AMV, A1MV)
|
Alfalfa mosaic virus (photo courtesy of A.J. Gibbs and VIDE) |
Other scientific names
Lucerne mosaic virus, Potato calico virus
Significance
Important virus disease of many forage legumes
Symptoms
Foliar yellow green mosaic, foliar malformation, twisting of leaflets, malformed and mosaic pods, chlorosis, systemic mosaic and mottling, stem necrosis, necrotic local lesions, proliferation of axillary shoots, dwarfing.
Hosts
Amaranthus retroflexus, Arachis hypogaea, Atriplex hortensis, Beta vulgaris, Brassica oleracea, Brassica rapa, Chenopodium capitatum, Chenopodium murale, Chenopodium quinoa, Chenopodium foetidum, Chenopodium album, Cicer arietinum, Crotalaria spectabilis, Cucumis sativus, Cyamopsis tetragonoloba, Glycine max, Lablab purpureus, Lotus corniculatus, Lupinus angustifolius, Lupinus albus, Macroptilium atropurpureum, Macroptilium lathyroides, Medicago sativa, Medicago polymorpha, Melilotus alba, Phaseolus lunatus, Phaseolus vulgaris, Pisum sativum, Senna tora, Sesbania herbacea, Trifolium incarnatum, Trifolium pretense, Trifolium repens, Trifolium subterraneum, Trifolium hybridum, Vicia sativa, Vicia villosa, Vigna radiata, Vigna unguiculata.
Geographic distribution
The virus is probably distributed worldwide.
Biology and transmission
Virus transmitted by a vector. Virus transmitted by mechanical inoculation; transmitted by grafting, not transmitted by contact between hosts; transmitted by seeds (50% in alfalfa seeds from individual infected plants and up to 10% in commercial seed, transmitted by pollen to the seed.
Virus transmitted by arthropods, by insects of the order Hemiptera, a family Aphididae; Myzus persicae, Aphis gossypii and at least 13 other species. Virus is transmitted in a non-persistent manner.
Detection/indexing method
- At CIAT: No significant importance
- At ILRI: ELISA, TBIA
- At ICARDA: ELISA, TBIA
Procedure followed at the centers in case of positive test
- Infected plants incinerated and fields rogued for plants with symptoms. If entire seed batch infected, regeneration started in insect proof area.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.). 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Allen DJ, Lenne JM. (eds.). 1998. The Pathology of Food and Pasture Legumes. CAB International, UK & International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India. 750 pp.
Gad L, Thottappilly G. (eds.). 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenne JM, Trutmann P. (eds.). 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & CIAT (Centro Internacional de Agricultura Tropical), Colombia. 404 pp.
National Bureau of Plant Genetic Resources. 1994. Checklist on Seed Transmitted Viruses: Leguminous Hosts. National Bureau of Plant Genetic Resources (NBPGR), India. 14 pp.
Sukumar C, Leath KT, Skipp RS, Pederson GA, Bray RA, Latch GMC, Jr Nutter FW. (eds.). 1996. Pasture and Forage Crop Pathology. American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc. 653 pp.
Bean common mosaic virus (BCMV)
|
Bean common mosaic virus (BCMV) (photo: CIAT) |
Other scientific names
Mungbean mosaic virus (MBRV), Bean common mosaic virus — serotype B,
Azuki bean mosaic virus, Bean mosaic virus, Bean western mosaic virus (Bos, 1964), Blackeye cowpea mosaic virus (305, mungbean mosaic virus (Abu Kassim, 1981; Kaiser and Mossahebi, 1974; Rao et al., 1986), Peanut stripe virus, Peanut mild mottle virus, Peanut chlorotic ring mottle virus, Sesame yellow mosaic virus
Significance
Widespread with high seed transmission rates
Symptoms
Leaf distortion and blistering, dwarfing, downward curling of leaf margins, vascular necrosis, light and dark green mosaic, ring-shaped & pin-point local lesions, distortion of flowers and buds.
Hosts
Arachis hypogaea, Bauhinia purpurea, Cajanus cajan, Centrosema pubescens, Chenopodium quinoa, Cicer arietinum, Clitoria ternatea, Crotalaria incana, Crotalaria juncea, Crotalaria spectabilis, Cucumis sativus, Cyamopsis tetragonoloba, Glycine max, Lablab purpureus, Lupinus angustifolius, Lupinus luteus, Lupinus albus, Macroptilium atropurpureum, Macroptilium lathyroides, Medicago sativa, Melilotus alba, Phaseolus acutifolius, Phaseolus lunatus, Phaseolus vulgaris, Pisum sativum, Rhynchosia minima, Senna sophera, Sena tora, Sesbania herbacea, Trifolium incarnatum, Trifolium pretense, Trifolium repens, Trifolium subterraneum, Trifolium hybridum, Vicia sativa, Vicia villosa, Vigna radiata, Vigna unguiculata, Vigna vexillata, Vigna subterranea.
Geographic distribution
Predominates in the Western World
The virus is probably distributed worldwide (in Phaseolus beans wherever they are grown). The virus occurs in China and the United States of America.
Biology and transmission
Virus transmitted by a vector. Virus is transmitted by mechanical inoculation (sap), transmitted by seeds (up to 83% in Phaseolus vulgaris and from 7-22% in tepary bean, transmitted by pollen to seed.
Virus transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; Acyrthosiphon pisum, Aphis craccivora, A. fabae, Myzus persicae and other species. Virus is transmitted in a non-persistent manner.
Bean common mosaic is caused by two species of the genus Potyvirus: Bean common mosaic virus (BCMV) and Bean common mosaic necrosis virus (BCMNV).
Detection/indexing method
- At CIAT: ELISA, kit for Potyvirus group
- At ILRI: ELISA, TBIA
- At ICARDA: ELISA, TBIA
Procedure followed at the centers in case of positive test
- Infected plants incinerated and fields rogued for plants with symptoms. If entire seed batch infected, regeneration started in insect proof area.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.). 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Allen DJ, Lenne JM. (eds.). 1998. The Pathology of Food and Pasture Legumes. CAB International, UK & International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India. 750 pp.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Gad L, Thottappilly G. (eds.). 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Lenne JM, Trutmann P. (eds.). 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & CIAT (Centro Internacional de Agricultura Tropical), Colombia. 404 pp.
National Bureau of Plant Genetic Resources. 1994. Checklist on Seed Transmitted Viruses: Leguminous Hosts. National Bureau of Plant Genetic Resources (NBPGR), India. 14 pp.
Olufemi WA, Mbiele AL, Nkouka N. (eds.). 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Sukumar C, Leath KT, Skipp RS, Pederson GA, Bray RA, Latch GMC, Jr Nutter FW. (eds.). 1996. Pasture and Forage Crop Pathology. American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc. 653 pp.
Bean southern mosaic virus (SBMV)
|
Bean southern mosaic virus (SBMV)(photo: CIAT) |
Other scientific names
Southern bean mosaic virus, Southern bean mosaic virus 1
Symptoms
Symptomless infection
Severe leaf mosaic and/or mottle; leaf deformity; light olive-green color of leaves; down curling of infected leaves; stunting
Hosts
Cassia tora, Cicer arietinum, Cyamopsis tetragonoloba, Glycine max, Lupinus albus, Melilotus albus, Phaseolus acutifolius, P. lunatus, P. vulgaris, Pisum sativum, Vigna mungo, V. radiata, V. subterranea, V. unguiculata, Vigna unguiculata ssp. sesquipedalis.
Geographic distribution
The virus spreads in Africa, North America, South and Central Americas and has also been found in France.
Biology and transmission
Virus is transmitted by a vector, sap, mechanical inoculation, grafting, seeds (3-7% in V. unguiculata cv.); transmitted by pollen to seed and transmitted by pollen to the pollinated plant.
Virus is transmitted by arthropods, by insects of the order Coleoptera; Chrysomelidae: Ceratoma trifurcate, Epilachna variestis. Virus is transmitted in a semi-persistent manner.
Cowpea resistance includes infection localization and inhibition of virus synthesis.
Detection/indexing method
- At CIAT: ELISA
- At ILRI: ELISA, TBIA
- At ICARDA: ELISA, TBIA
Procedure followed at the centers in case of positive test
- Infected plants incinerated and fields rogued for plants with symptoms. If entire seed batch infected, regeneration started in insect proof area.
References and further reading
Allen DJ, Lenne JM. (eds.). 1998. The Pathology of Food and Pasture Legumes. CAB International, UK & International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India. 750 pp.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Gad L, Thottappilly G. (eds.). 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
National Bureau of Plant Genetic Resources. 1994. Checklist on Seed Transmitted Viruses: Leguminous Hosts. National Bureau of Plant Genetic Resources (NBPGR), India. 14 pp.
Bean yellow mosaic virus (BYMV)
Other scientific names
Pea mosaic virus, Bean virus 2, Canna mosaic virus (Brierley and Smith, 1948), Gladiolus mosaic virus, Gloriosa stripe mosaic virus
Significance
Widespread.
Symptoms
Clearing of small veins in young leaves, intense yellow mottling, rusty necrotic spots in yellow areas, crinkling of leaves, stunting, contrasting yellow and green areas in leaves, yellow mosaic, rugosity, malformation.
Hosts
Amaranthus retroflexus, Arachis hypogaea, Cajanus cajan, Canavalia ensiformis, Centrosema pubescens, Chenopodium murale, Chenopodium quinoa, Chenopodium album, Cicer arietinum, Crotalaria juncea, Crotalaria pallida, Crotalaria retusa, Crotalaria spectabilis, Cucumis sativus, Cyamopsis tetragonoloba, Glycine max, Lupinus angustifolius, Lupinus luteus, Lupinus albus, Macrotyloma uniflorum, Medicago sativa, Melilotus alba, Phaseolus lunatus, Phaseolus vulgaris, Phaseolus coccineus, Pisum sativum, Psophocarpus tetragonolobus, Senna tora, Trifolium incarnatum, Trifolium pretense, Trifolium repens, Trifolium resupinatum, Trifolium michelianum, Trifolium subterraneum, Trifolium hybridum, Trigonella foenum-graecum, Vicia sativa, Vicia villosa, Vigna radiata, Vigna unguiculata.
Geographic distribution
The virus is probably distributed worldwide.
Biology and transmission
Virus transmitted by mechanical inoculation; transmitted by seeds (to 3%); transmitted by seed, sap and mechanical inoculation; seed-borne in Lupinus albus, L. luteus, Trifolium pratense, Vicia faba.
Virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; more than 20 ssp. including Acyrthosiphon pisum, Macrosiphum euphorbiae, Myzus persicae, Aphis fabae. Virus is transmitted in a non-persistent manner.
Detection/indexing method
- At CIAT: No significant importance
- At ILRI: ELISA, TBIA
- At ICARDA: ELISA, TBIA
Procedure followed at the centers in case of positive test
- Infected plants incinerated and fields rogued for plants with symptoms. If entire seed batch infected, regeneration started in insect proof area.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.). 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Allen DJ, Lenne JM. (eds.). 1998. The Pathology of Food and Pasture Legumes. CAB International, UK & International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India. 750 pp.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Gad L, Thottappilly G. (eds.). 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Lenne JM, Trutmann P. (eds.). 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & CIAT (Centro Internacional de Agricultura Tropical), Colombia. 404 pp.
National Bureau of Plant Genetic Resources. 1994. Checklist on Seed Transmitted Viruses: Leguminous Hosts. National Bureau of Plant Genetic Resources (NBPGR), India. 14 pp.
Olufemi WA, Mbiele AL, Nkouka N. (eds.). 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Sukumar C, Leath KT, Skipp RS, Pederson GA, Bray RA, Latch GCM, Jr Nutter FW. (eds.). 1996. Pasture and Forage Crop Pathology. American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc. 653 pp.
Cowpea mosaic virus (CPMV or CpMV)
Other scientific names
Cowpea yellow mosaic virus (CYMV), Cowpea mosaic yellow strain virus (CMYSV), Cowpea mosaic virus, SB isolate
Significance
Important in SSA.
Symptoms
Vein clearing and necrosis, leaf distortion, lamina necrosis, light green mottle, necrotic local lesions, systemic chlorotic spots and streaks, apical deformation, pinpoint necrotic local lesions.
Hosts
Arachis hypogaea, Beta vulgaris, Cajanus cajan, Canavalia ensiformis, Centrosema pubescens, Chenopodium quinoa, Crotalaria juncea, Crotalaria retusa, Crotalaria spectabilis, Cucumis sativus, Cyamopsis tetragonoloba, Glycine max, Lablab purpureus, Lupinus albus, Macrotyloma uniflorum, Medicago sativa, Melilotus alba, Phaseolus lunatus, Phaseolus vulgaris, Pisum sativum, Psophocarpus tetragonolobus, Senna tora, Trifolium incarnatum, Trifolium pretense, Trifolium repens, Vicia sativa, Vicia villosa, Vigna radiata, Vigna unguiculata, Vigna subterranea.
Geographic distribution
Spreads in Cuba, Kenya, Nigeria, Suriname, and Tanzania. Found, but with no evidence of spread, in the USA.
Biology and transmission
Vector: Beetle
Transmitted by seed and sap
Mechanical transmission by grafting
Detection/indexing method
- At CIAT: No significant important
- At ILRI: ELISA, TBIA
- At ICARDA: ELISA, TBIA
Procedure followed at the centers in case of positive test
- Infected plants incinerated and fields rogued for plants with symptoms. If entire seed batch infected, regeneration started in insect proof area.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.). 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Allen DJ, Lenne JM. (eds.). 1998. The Pathology of Food and Pasture Legumes. CAB International, UK & International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India. 750 pp.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenne JM, Trutmann P. (eds.). 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & CIAT (Centro Internacional de Agricultura Tropical), Colombia. 404 pp.
Olufemi WA, Mbiele AL, Nkouka N. (eds.). 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Peanut mottle virus (PeMoV, PMoV or PnMV)
Other scientific names
Groundnut mottle virus, Peanut mild mosaic virus, Peanut severe mosaic virus.
Symptoms
Yellow vein clearing, crinkled leaves, blistered leaves, ring foliar lesions, variegation, deformed leaves, mottling with necrosis and mosaic chlorotic ringspot in leaves, mild leaf chlorosis and stunting.
Hosts
Amaranthus retroflexus, Arachis hypogaea, Arachis pintoi, Beta vulgaris, Brassica rapa, Cajanus cajan, Chenopodium murale, Chenopodium quinoa, Chenopodium album, Citrullus lanatus, Crotalaria spectabilis, Cucumis sativus, Cyamopsis tetragonoloba, Glycine max, Lupinus angustifolius, Lupinus albus, Macroptilium lathyroides, Medicago sativa, Melilotus alba, Melilotus officinalis, Phaseolus acutifolius, Phaseolus lunatus, Phaseolus vulgaris, Pisum sativum, Senna bicapsularis, Senna obtusifolia, Senna occidentalis, Senna tora, Trifolium incarnatum, Trifolium pretense, Trifolium repens, Trifolium subterraneum, Trifolium hybridum, Vicia villosa, Vigna unguiculata, Vigna subterranean.
Geographic distribution
The virus spreads in Africa, East Asia, and South and Central Americas. The virus occurs in Australia (the north east), or Colombia, or India (possibly), or Japan, or Malaysia, or the Philippines, or Taiwan, or the United States of America (in the south east).
Biology and transmission
Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by seeds (0.02-2% in Arachis hypogaea; to 1% in Phaseolus vulgaris and to 0.008% in Vigna unguiculata (Demski et al., 1983), but not in Glycine max, Pisum sativum, Cassia obtusifolia).
Virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; Aphis craccivora, A. gossypii, Hyperomyzus lactucae, Myzus persicae, Rhopalosiphum padi. Virus is transmitted in a non-persistent manner (A. craccivora can remain infective for 2 hours and M. persicae for 12 hours after acquisition).
Seed transmission in peanuts and beans, mottle and necrosis in peanut, bean and pea, Arachis glabrata – resistant.
Detection/indexing method
- At CIAT: ELISA, kit for Potyvirus group
- At ILRI: ELISA, TBIA
- At ICARDA: ELISA, TBIA
Procedure followed at the centers in case of positive test
- Infected plants incinerated and fields rogued for plants with symptoms. If entire seed batch infected, regeneration started in insect proof area.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.). 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Allen DJ, Lenne JM. (eds.). 1998. The Pathology of Food and Pasture Legumes. CAB International, UK & International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India. 750 pp.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Gad L, Thottappilly G. (eds.). 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Lenne JM, Trutmann P. (eds.). 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & CIAT (Centro Internacional de Agricultura Tropical), Colombia. 404 pp.
National Bureau of Plant Genetic Resources. 1994. Checklist on Seed Transmitted Viruses: Leguminous Hosts. National Bureau of Plant Genetic Resources (NBPGR), India. 14 pp.
Olufemi WA, Mbiele AL, Nkouka N. (eds.). 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
 PeMoV in Arachis pintoi and Macroptilium lathyroides (photos:URG-CIAT) |
Soybean mosaic virus (SMV or SbMV)
Symptoms
Leaves crinkled, curl longitudinally downward, slight mottle, leaves raised or blistered along main veins, browning in stems and petioles, defoliation, stunted with fewer pods (flat, no hairs and seeds), systemic necrosis, chlorosis develop between dark and green areas of the leaves, seeds mottled brown or black
Hosts
Beta vulgaris, Brassica rapa, Cajanus cajan, Canavalia ensiformis, Centrosema brasilianum, Centrosema macrocarpum, Centrosema pascuorum, Centrosema pubescens, Centrosema acutifolium, Chenopodium quinoa, Chenopodium album, Crotalaria spectabilis, Cucumis sativus, Cyamopsis tetragonoloba, Glycine max, Indigofera hirsuta, Kummerowia striata, Lablab purpureus, Lotus corniculatus, Lotus tetragonolobus, Lotus corniculatus, Lupinus angustifolius, Lupinus luteus, Lupinus albus, Macroptilium lathyroides, Macrotyloma uniflorum, Medicago sativa, Medicago alba, Melilotus officinalis, Phaseolus lunatus, Phaseolus vulgaris, Pisum sativum, Senna occidentalis, Senna tora, Sesbania herbacea, Trifolium incarnatum, Trifolium pretense, Trifolium repens, Trifolium hybridum, Trigonella foenum-graecum, Vigna radiata, Vigna unguiculata
Geographic distribution
The virus is probably distributed worldwide.
Biology and transmission
Virus is normally transmitted by a vector. Virus is transmitted by mechanical inoculation (sap), grafting; up to 30% or higher transmission by seeds (not in Glycine max cvs Kawanggyo, Hill or Bienville), transmitted by pollen to the seed, or transmitted by pollen to the pollinated plant.
Virus is transmitted by arthropods, by insects of the order Hemiptera, family Aphididae; 16 species, including Acyrthosiphon pisum, Aphis fabae, Myzus persicae. Virus is transmitted in a non-persistent manner; does not require a helper virus for vector transmission (One strain is not aphid transmitted).
Most widely distributed virus of soybean; Calopogonium sp. – natural reservoir.
Detection/indexing method
- At CIAT: ELISA, kit for Potyvirus group
- At ILRI: ELISA, TBIA
- At ICARDA: ELISA, TBIA
Procedure followed at the centers in case of positive test
- Infected plants incinerated and fields rogued for plants with symptoms. If entire seed batch infected, regeneration started in insect proof area.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.). 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Allen DJ, Lenne JM. (eds.). 1998. The Pathology of Food and Pasture Legumes. CAB International, UK & International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), India. 750 pp.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Gad L, Thottappilly G. (eds.). 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Lenne JM, Trutmann P. (eds.). 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & CIAT (Centro Internacional de Agricultura Tropical), Colombia. 404 pp.
National Bureau of Plant Genetic Resources. 1994. Checklist on Seed Transmitted Viruses: Leguminous Hosts. National Bureau of Plant Genetic Resources (NBPGR), India. 14 pp.
Olufemi WA, Mbiele AL, Nkouka N. (eds.). 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.057.0.01.061.htm
Symptoms
Vein-clearing, mosaic, leaf malformation
 Centrosema mosaic virus (photo: Virology Unit, CIAT) |
Hosts
Centrosema pubescens, Crotalaria anagyroides, C. retusa, C. goreensis, C. mucronata, Calopogonium mucunoides (virus causes lack of seed set), Desmodium distortum.
Geographic distribution
The virus occurs in Papua New Guinea, Colombia and Caribean area
Biology and transmission
Virus is a tentative member of the genus Potyvirus in the family Flexiviridae. Virus is transmitted by arthropods, by insects of the order Heteroptera; Lygaeidae and Nysius ssp. Virus is transmitted in a non-persistent manner, and demonstrated seed transmissibility in Centrosema spp.
Detection/indexing method
- At CIAT: ELISA, kit for Potyvirus group
Procedure followed at the center in case of positive test
- Infected plants incinerated and fields rogued for plants with symptoms. If entire seed batch infected, regeneration started in insect proof area.
References and further reading
Morales FJ, Virologist AI, Castaño NM, Calvert L. Centro Internacional de Agricultura Tropical, Cali, Colombia. Detection of a Strain of Soybean Mosaic Virus Affecting Tropical Forage Species of Centrosema. Plant Dis. 74:648-651
Schultze-Kraft R, Clements RJ, Keller-Grein G. Centrosema: Biologia, Agronomia y utilizacion. Publicado por Centro Internacional de Agricultura Tropical, 1997
van Velsen RJ, Crowley NC. 1962. Centrosema Mosaic: A new virus disease of Crotalaria spp. In Papua and New Guinea. Aust. J. Agric. Res. 13:220-232
Nematodes - forage legume
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell); ICARDA, Syria (Siham Asaad).
|
Contents: |
Stem and bulb nematode infection
Scientific name
Ditylenchus dipsaci (Kühn) Filipjev
Significance
A major pest in temperate climates and high-altitude regions of the tropics and subtropics.
Symptoms
Plants become distorted and stunted; infected tissues are spongy; damage can predispose plants to other problems.
Field shows irregular areas of sparse growth.
Clover and alfalfa show reduction of internode length and swollen stems.
Hosts
More than 400 host plants have been described for D. dipsaci. The species is subdivided in races. The Allium race also attacks oats, sugar beet, Swiss chard, spinach and legumes (fababean, bean, pea, soybean).
Medicago sativa, Glycine max, Trifolium spp.
Geographic distribution
Cosmopolitan, except tropical lowlands; temperate regions where it is one of the most devastating plant parasites; USA.
Biology and transmission
The nematode is carred inside the seed, and it is a seed and soil borne nematode.
Nematode is a migratory endoparasite. At the beginning of the crop season, 4th-stage juvenile enters young tissues, especially seedlings when below the soil surface. Feeding breaks down middle lamellae; nematode probably secretes a pectinase enzyme; plant parts become ‘crisp’ and are easily broken. Migration on plant parts above ground requires free water, and may occur after rain or sprinkler irrigation. Nematode enters through stomata or by direct penetration.
Cardinal temperatures for nematode activity and infection are 10oC - 22oC -30oC. In soil, they survive as fourth stage larvae at temperatures not exceeding 35oC.
Infestation occurs readily in heavier soils and during times of high rainfall or in sprinkler-irrigated areas.
Nematode is spread around field by equipment, irrigation; spreads readily in tail water.
Predisposes alfalfa to Phytophthora megasperma.
Detection/indexing method
- At CIAT: No significant importance
- At ICARDA:, Nematode extraction
References and further references
http://plpnemweb.ucdavis.edu/Nemaplex/Taxadata/G042S1.HTM#Distribution:
Diekmann M. 1997. FAO/IBPGR Technical guidelines for the safe movement of Germplasm. No. 18. Allium spp. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Root-knot nematode infestation
Scientific Name
Meloidogyne spp.
Symptoms
Root knot nematode infestation includes wilting, loss of vigor, yellowing and other symptoms similar to a lack of water or nutrients. Plants often wilt during the hottest part of the day, even with adequate soil moisture, and leaves may turn yellow. Fewer and smaller leaves and fruits are produced, and plants heavily infested early in the season may die.
Root knot nematodes usually cause distinctive swellings, called galls, on the roots of affected plants. Infestations of these nematodes are fairly easy to recognize by digging up a few plants with symptoms, washing or gently tapping the soil from the roots, examining the roots for galls. The nematodes feed and develop within the galls, which may grow to as large as 1 inch in diameter on some plants but are usually much smaller. The water- and nutrient-conducting abilities of the roots are damaged by the formation of the galls. Galls may crack or split open, especially on the roots of vegetable plants, allowing the entry of soil-borne, disease-causing microorganisms.
Root knot nematode galls are true swellings and cannot be rubbed off the roots, as can the beneficial nitrogen-fixing nodules on the roots of legumes.
Hosts
Root knot nematodes have a broad host range of more than 200 reported plants.
As a genus, they are reported as parasites of over 3000 host plants, and individual species often have a wide host range. Some 874 crop species act as hosts of the 7 or 8 species commonly occurring in the western U.S.
Geographic distribution
Southeast Asia, South America, USA
Biology and transmission
The root-knot nematodes (Meloidogyne spp.) form easily recognized galls on the roots. Galls result from growth of plant tissues around juvenile nematodes which feed near the centre of the root. Root-knot gall tissue is firm without a hollow centre, and is an integral part of the root; removing a root-knot gall from a root tears root tissue.
They are difficult to control and can be spread easily from garden to garden in soil (for example, on tools, boots, etc.) and plant parts.
Certain marigolds (Tagetes) suppress root knot nematodes. The effect of marigolds is greatest when they are grown as a solid planting for an entire season. When grown along with annual vegetables or under trees or vines (intercropping), nematode control is usually not very good. As with other cultural control methods, nematode populations will rapidly increase as soon as susceptible crops are grown.
Root knot nematodes may feed on the roots of grasses and certain legumes without causing galling.
Damage is most serious in warm, irrigated, sandy soils.
Detection/indexing method
- At CIAT: Visualization in Stereomicroscopy and Microscopy
Procedure followed at the center in case of positive test
- Species rotation in regeneration fields.
References and further reading
http://plantclinic.cornell.edu/FactSheets/nematodes/nematodes.htm
http://www.ipm.ucdavis.edu/PMG/PESTNOTES/pn7489.html
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.