Stog-sorghum
Mites - sorghum
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Weeds (sorghum)
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Viruses (sorghum)
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Insects - sorghum
Contributors to this page: RP Thakur, AG Girish and VP Rao
|
Contents: |
|
Rice Meal Moth (Corcyra cephalonica) of sorghum: (A)adult and (B)larvae in infested grain and C. pupa in infested grain (photos: ICRISAT). |
Scientific name
Corcyra cephalonica Stainton.
Importance
Low
Significance
In one instance, it was found that the weight loss of some infested rice was 7%, whilst the cost of cleaning the grain to make it saleable amounted to 9.4% of the value of the original stock
Symptoms
Infested produce is densely webbed.
Hosts
Oryza sativa (rice), Manihot esculenta (cassava), Myristica fragrans (nutmeg), Panicum miliaceum (wild millet), Pennisetum glaucum (pearl millet), Triticum (wheat), Triticum aestivum (wheat), Zea mays (maize). Arachis hypogaea (groundnut), Cajanus cajan (pigeon pea), Cicer arietinum (chickpea), Capsicum annuum (bell pepper), Ficus carica (common fig), Gossypium (cotton), Vigna radiata (bean, mung), Prunus armeniaca (apricot), Sesamum indicum (sesame), Theobroma cacao (cocoa) and Vigna unguiculata (cowpea).
Geographic distribution
It is a major storage pest in India, Thailand, Brazil, Ghana, Myanmar, Sri Lanka and Indonesia and several countries in Africa where major cereal crops are rice, maize, sorghum and other cereals.
Biology and transmission
The adult moth is a pale grayish brown, with a wing expanse of 14-24 mm, and the head bears a projecting tuft of scales. The adult moths are short lived (Teetes et al. 1983).
The eggs are spherical and white and up to 200 are laid loose in food material. The incubation period is 3-5 days.
Larvae are creamy white and Webb together particles of food and frass with silken threads into galleries in which they live and feed. The larval period is 20-30 days and pupal period 9-10 days.
Detection/indexing methods
- Agar plate method
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed seed treatment with chlorpyriphos 3 g kg-1 seed (Ghanekar et al. 1996).
Procedures followed at the centers in case of positive test
- Highly infested samples are rejected
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24(1-2): 37-43.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P., India: International Crops Research Institute for Semi-Arid Tropics. 125pp.
Scientific name
Ephestia cautella Walker.
Other scientific names
Cadra cautella
Importance
Low
Significance
Extensive resources are used to control this pest species in industrial flour mills (Nielsen 2000).
Symptoms
Damage always begins on the outside surface of grains or packaging, if these are sufficiently friable to be pierced and then cut by the mandibles of the first-instars larvae. Dried or partly dried fruits have fissures, which are either natural (such as the hilum) or caused by other means (entry or exit holes made by other primary insect pests or damage caused by rodents). These fissures serve as feeding and anchoring points for the construction of the pupation cocoon, if there is enough space for good air circulation. Thus damage may be located on the edges of the stores, whether they have already been infested (the surviving caterpillars search for light and air) or during new infestations.
Host
It is a storage pest that affects many cereals including Sorghum bicolor (sorghum), Triticum aestivum (wheat), Zea mays (maize), Oryzae sativa (rice), Avena sativa (oats) and Hordeum vulgare (barley). It also attacks Arachis hypogaea (groundnut), Ceratonia siliqua (locust bean), Cannabis sativa (hemp), Capsicum (peppers), Glycine max (soybean), Juglans (walnuts) (rice), Phoenix dactylifera (date-palm), Pisum sativum (pea), Prunus (stone fruit), Prunus dulcis (almond), Sesamum (Sesame), Solanum tuberosum (potato) and Theobroma cacao (cocoa).
Geographic distribution
Cosmopolitan
Biology
The adult is grayish in color with transverse strips on its outer wings, while at rest fore part is elevated giving a distinct slope to the wings which are wrapped abut the body. Wings expanse is less than 25 mm. The females indiscriminately lay small whitish eggs in the store in cracks in craves end on the food itself. It lays about 250 eggs. The eggs hatch in about 3 days and the young larva spill silk profusely and spin small silken tubes among the food particles. The full-grown larva is 2-12 mm long, white in color with a pinkish tinge, larval period last from 40-50 days depending upon the temperature. The full-grown larvae spin cocoons and pupate there in. The pupal period lasts for about 12 days or so. The complete life cycle takes about 2 months and thus 5 or 6 generations in a year.
Detection/indexing methods at ICRISAT
- Dry seed examination
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed seed treatment with chlorpyriphos 3 g kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Dry seed examination
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Nielsen PS. 2000. Alternatives to methyl bromide. IPM in flourmills; comparison of a Norwegian and Danish mill. TemaNord2000: 510. Nordic Council of Ministers, Copenhagen, Denmark.
 Almond Moth (Ephestia cautella) (A)adult and (B)larva and pupa of wit frass (photos:www.UKmoths.org.UK) |
Scientific name
Oryzaephilus surinamensis Linnaeus.
Other scientific names
Anobium frumentarium, Dermestes sexdentatu , Dermestes surinamensis
Importance
Low
Significance
The sawtoothed grain beetle is a secondary pest that attacks damaged grain. It infests a wide range of grain-like products, from rice to corn flakes to birdseed to pancake flour to tapioca. However, infestations by these pests can lead to substantial contamination with frass and dead bodies. Thus, quality deterioration is an important issue.
Symptoms
It is a typical secondary pest, attacking previously damaged or broken kernels to feed, especially on the germ. The larvae also attack the germ in whole cereal grains, thereby altering the nutritional content and reducing the percentage germination.
 Sawtooted Grain Beetle (photo: commons.wikimedia.org) |
Hosts
Avena sativa (oats), Hordeum vulgare (barley), Myristica fragrans (nutmeg), Oryza sativa (rice), Panicum miliaceum (millet), Triticum (wheat), Zea mays (maize),Arachis hypogaea (groundnut), Azadirachta indica (bastard tree), Ceratonia siliqua (locust bean), Helianthus annuus (sunflower), Panicum (millets), Secale cereale (rye) and Vicia faba (broad bean).
Geographic distribution
Cosmopolitan
Biology and transmission
The sawtoothed grain beetle is a slender, dark brown beetle 2.4–3 mm in size, with characteristic teeth running down the side of the prothorax. The antennae are relatively short and weakly clubbed, and the prothorax has six distinctive tooth-like projections along each side. Eggs are laid singly or in small batches in some crevice in the food packs. The larvae are white, elongate, somewhat flattened and about 4-5 mm long when fully grown; with a pair of abdominal prolegs in addition to the 6 true legs on the thorax. The larval cycle is 27-35 days, with 2-4 molts depending on temperature (Mallis 1990).
Detection/indexing methods at ICRISAT
- None
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos 3 g kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Infested samples are rejected
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection. 24: 37-43.
Mallis A. 1990. Handbook of Pest Control. 7th edition. Cleveland: Franzak &Foster Co., pp. 524-6.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P., India: International Crops Research Institute for Semi-Arid Tropics. 125pp.
Scientific name
Plodia interpunctella Hubner.
Importance
Medium
Significance
Indian Meal Moth is a common grain-feeding pest found around the world, feeding on cereals and dry grain products.
Symptoms
Grains on the surface are often held together by a mat of silken webbing containing frass and larval skins. The larvae move through the commodity leaving a trail of webbing and excreta. White, silken cocoons containing the pupae can be seen on the sides of infested bags.
Biology and transmission
Female moths lay between 60 and 400 eggs (Lyon 1991) on a food surface, which are ordinarily smaller than 0.5 mm and not sticky. The eggs hatch in 2 to 14 days. The moth larvae are off-white with brown heads. When these larvae mature, they are usually about 12 mm long. The larval stage lasts from 2 to 41 weeks, depending on the temperature. Adult moths are 8–10 mm in length with 16–20 mm wingspans. The outer half of their forewings are bronze, copper, or dark gray in color, while the upper half are yellowish-gray, with a dark band at the intersection between the two. The entire life cycle may range from 30 to 300 days.
 Indian Meal Moth (photo:ICRISAT) |
Hosts
Arachis hypogaea (groundnut), Oryza sativa (rice), Prunus (stone fruit), stored products (dried stored products), Triticum aestivum (wheat), Zea mays (maize), Avena sativa (oats), Helianthus annuus (sunflower), Hordeum vulgare (barley), Juglans regia (Carpathian walnut), Pistacia vera (pistachio), Prunus dulcis (almond) and Theobroma cacao (cocoa).
Geographic distribution
Plodia interpunctella is cosmopolitan in distribution especially in warm climates. In cool temperate countries, it can survive in heated buildings.
Detection/indexing methods at ICRISAT
- None
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos 3 g kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Infested samples are rejected
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK and Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A and Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Lyon WF. 1991."Fact Sheet: Indianmeal Moth". Ohio State University. http://ohioline.osu.edu/hyg-fact/2000/2089.html. Retrieved on 2006-09-07.
Scientific name
Rhyzopertha dominica Fabricius.
Importance
Low
Significance
Rhyzopertha dominica is a pest of several stored products. It is a major pest in wheat and rice. The larvae and adults consume the seed. Infestations of R. dominica adversely affectsquantity and quality of stored seed.
Symptoms
Damaged grains with small holes can be seen under stereo binocular or with a magnifying lens.
Hosts
Avena sativa (oats), Hordeum vulgare (barley), Oryza sativa (rice), Panicum (millets), Pennisetum (feather grass), Triticum aestivum (wheat), Zea mays (maize), Coriandrum sativum (coriander), Capsicum frutescens (chilli), Curcuma longa (turmeric), Manihot esculenta (cassava), Phaseolus (beans) and Zingiber officinale (ginger).
Geographic distribution
Rhyzopertha dominica is cosmopolitan in its distribution and infestation can be severe.
Biology and transmission
The beetle is small (4 mm long), slender, cylindrical, polished dark-brown or black with a roughened wing surface. The head is turned down and covered by hood-shaped thorax that bears small patches around the edge. The female lays 300-500 eggs singly or in clusters on grains or in powdery material over a period of 3-6 weeks. In 5-11 days eggs hatch into fleshy grubs which appears swollen at the extremities. Grubs bore into grains and feed inside. The larval period lasts from 25 to 50 days depending on the season. Pupation takes place within the grain. The pupal period lasts 7-8 days. The total life cycle may take place in 2 months and 3-4 generations in a year (Teetes et al. 1983).
Detection/indexing methods at ICRISAT
- The simplest method is to sieve a 200-1000 g sample of the grain and look for adults as dry seed examination.
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos 3 g kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Infested samples are rejected
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P. , India: International Crops Research Institute for Semi-Arid Tropics. 125pp.
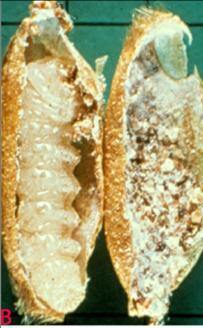
 Lesser Grain Borer (Rhizopertha dominica) of sorghum: (A) adult and (B) larva in the grain(photos: ICRISAT) |
Scientific name
Sitophilus oryzae Linnaeus.
Other scientific names
Sitophilus zeamais
Importance
Low
Significance
Moderate loss in sorghum grains in large and small grains. Threshed grain is more susceptible than unthreshed grain, and more progeny produced on threshed than on unthreshed sorghum.
Geographical distribution
Rice weevil is the most destructive pest of stored grain worldwide, and are cosmopolitan in distribution, but much more injurious in warm and humid countries (Teetes et al. 1983).
Symptons
Both the adults and larvae feed on grain, which may often be damaged beyond use.
Host
Oryza sativa (rice), Manihot esculenta (cassava), Triticum aestivum (wheat), Triticum spelta (spelt), Zea mays (maize), Cicer arietinum (chickpea), Hordeum vulgare (barley), Lens culinaris (small seeded lentil), Panicum (millets), Pennisetum glaucum (pearl millet), Vigna angularis (adzuki bean), Vigna radiata (mung bean ), Pisum sativum (pea), Secale cereale (rye) and Vigna unguiculata (cowpea).
Biology
Adult weevils are reddish brown; about 4mm long, and have four light reddish or yellowish spots on the wings. The adult female bores a hole in a kernel, deposits a single egg and covers it with a gelatinous fluid. The female may lay 300-550 eggs in 4-5 months. The incubation period is 3 days. The grub is legless, short, stout and whitish with a brown head. A larva matures in 3-6 days. The longevity of the adult is 4-5 months (Teetes et al. 1983).
Detection/indexing methods at ICRISAT
- Dry seed examination
Treatments/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos at 3 g kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
- Removal of the infested seed if it is less than 5%.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection. 24: 37-43.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P., India: International Crops Research Institute for Semi-Arid Tropics. 125pp.
 Rice Weevil (Sitpphilus oryzae) of sorghum: (A)adult and (B)infested grain (photos:ICRISAT) |
Scientific name
Sitotroga cerealella Olivier.
Other scientific names
Tinea hordei
Importance
Medium
Significance
An important stored pest of sorghum and can cause losses of up to 50% during storage. High temperature and poor storage hygiene are major factors resulting in insect infestation (Seifelnasr 1992).
Symptoms
Infestation can begin in the fields. In storage, the infestation is confined to the upper layer of the grains. The larva bores into the grain and remains there until emerges as an adult from round emergence holes. The infested grain is completely hallowed out and filled with larval excreta.
Hosts
Avena sativa (oats), Hordeum vulgare (barley), Oryza sativa (rice), Pennisetum glaucum (pearl millet), Secale cereale (rye), Triticum aestivum (wheat), Triticum spelta (spelt) and Zea mays (maize).
Geographic distribution
It is cosmopolitan in distribution. It is one of the primary pests of the stored sorghum and rice grain, but is also known to attack maize, wheat, and barley.
Biology
The female can lay up to 400 eggs that are deposited indiscriminately on or between the grains, on the panicles in the field, or in the storage. The egg is white and oval, but soon turns bright red and hatches within a week. The tiny larva crawls about searching for a comparatively weak spot through which it enters the grain, and feeds on the internal contents. The larval period is 2-3 weeks. The moth emerges within a week (Teetes et al. 1983).
Detection/indexing methods at ICRISAT
- Dry seed examination
Treatment/control
- Fumigation with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos at 3 g kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test at ICRISAT
Highly infested samples are rejected
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Seifelnasr YE.1992. Farmers’perceptions on management practices of insect pests on stored sorghum in southwestern Ethiopia. Crop Protection, 26: 1817-1825.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P., India: International Crops Research
Institute for Semi-Arid Tropics. 125pp.
CGIAR-published references on seed health
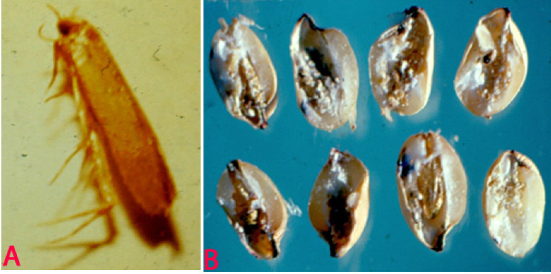 Angoumois Grain Moth (Sitotroga cerealella) of corghum: (A)adult and (B)infested grains (photos:ICRISAT) |
Scientific name
Prostephanus truncatus Horn.
Synonyms
Dinoderus truncatus
Importance
Low
Significance
A highly damaging pest of sorghum causing upto 20% grain loss (Mailafiya et al. 2008).
Symptoms
Adults frequently initiate their attack on stored seed with intact sheaths by boring into the base of seed. Adults bore into the grains, making neat round holes, and as they tunnel from grain to grain they generate large quantities of grain dust. Adult females lay eggs in chambers bored at right angles to the main tunnels.
 Larger Grain Borer (Prostephanus truncatus) (photo:www.agrsci.dk) |
Hosts
Manihot esculenta (cassava), stored products (dried stored products), Zea mays (maize), Dioscorea (yam) and Triticum aestivum (wheat).
Geographic distribution
Prostephanus truncatus is indigenous to Central America, tropical South America, and the extreme south of the USA as a major pest. It is also distributed in Africa and Europe, but restricted distribution in India.
Biology
The adult has the typical cylindrical bostrichid shape with body length of 3-4.5 mm. The declivity is flattened and steep and has many small tubercles over its surface. The limits of the declivity, apically and laterally, are marked by a carina. The antennae are 10-segmented and have a loose three-segmented club; the 'stem' of the antenna is slender and clothed with long hairs and the apical club segment is as wide as, or wider than, the preceding segments. The larvae are white, fleshy and sparsely covered with hairs. They are parallel-sided, i.e. they do not taper. The legs are short and the head capsule is small relative to the size of the body.
Detection/indexing methods at ICRISAT
- Not detected.
Treatment/control
- Not suggested due to non-detection of the pest.
Procedures followed in case of positive test at ICRISAT
Highly infested samples are rejected
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Mailafiya DM, Ayertey JN, Cudjoe AR. 2008.Damage and weight loss potential of Prostephanus truncatus Horn Coleoptera: Bostrichidae on sorghum grain: implication to cereal grain storage in sub-Saharan Africa. International Journal of pure and applied sciences 2: 28-35.
CGIAR-published references on seed health
Scientific name
Tribolium castaneum Herbst.
Importance
Medium
Significance
Important in stored sorghum grain in several countries.
Symptoms
Infestation by adult beetles can be readily observed by the tunnels they leave when they move through the flour and other granular food products. Damage is particularly serious in grains such as rice and wheat, which have either been dehusked or processed into other products. When infestation is severe, these products turn grayish-yellow and become moldy, with a pungent odor. Infestation may also be apparent by the appearance of adults on the surface of the grains (Teetes et al. 1983).
Hosts
Arachis hypogaea (groundnut), Avena sativa (oats), Bertholletia excelsa (Brazil nut), Hordeum vulgare (barley), Juglans (walnuts), Lens culinaris spp. Culinaris (lentil), Oryza sativa (rice), Phaseolus (beans), Phaseolus lunatus (lima bean), Pisum sativum (pea), Prunus dulcis (almond), Secale cereale (rye), Triticum (wheat), Triticum spelta (spelt) and Zea mays (maize).
Geographic distribution
Tribolium castaneum is cosmopolitan; found infesting stored grain, seeds, flour, dried fruits, nuts (Teetes et al. 1983).
Biology and transmission
The adult is 2.3-4.4 mm long, rather flat, oblong and chestnut-brown (reddish-brown). It lays about 450 eggs in stored produce. The eggs of are minute, cylindrical and white. The incubation period lasts for 5-12 days. The yellowish-white cylindrical grub is covered with fine hairs. The pupa is naked (without a cocoon), yellowish-white, becoming brown later, and adults emerge in 3-7 days (Teetes et al 1983).
Detection/indexing methods at ICRISAT
- X-ray radiography is used for suspected samples because it offers a non-destructive method. Dry seed examination using magnifying lens to separate the infested seed.
Treatment/control
- Fumigation of the samples with methyl bromide by 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos at 3g kg-1 seed (Ghanekar et al. 1996).
Procedures followed in case of positive test
- Infested samples are rejected.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P., India: International Crops Research
Institute for Semi-Arid Tropics. 125pp.
 Red Flour Beetle (Tribolium castaneum) of sourghum: (A)adult and (B)infested grains (photos:ICRISAT) |
Significance
Trogoderma granarium Everts.
Other scientific names
Trogoderma afrum, Trogoderma khapra, Trogoderma quinquefasciata
Importance
Medium
Significance
Trogoderma granarium is a serious pest of stored products under hot dry conditions. Established infestations are difficult to control because of the beetle's ability to live without food for long periods of time and to survive on foods of low moisture content, its habit of crawling into tiny cracks and crevices and remaining there for long periods, and its relative tolerance to many surface insecticides and fumigants.
Symptoms
The khapra beetle is one of the world's most feared stored-product pests. The obvious signs of a khapra beetle infestation are the larvae and cast skins. Larvae and adults are best identified by microscopic examination. Larvae are mostly seen just before dusk, since they are more active at that time (Anonymous 1981).
Host
Larvae feed on a wide variety of stored products and dried foods. They prefer whole grain and cereal products such as Triticum aestivum (wheat), Hordeum vulgare (barley), and Oryza sativa(rice), but larvae have been recorded on the following: Avena spp. (oats), Secale spp. (rye), Zea mays (corn), dried blood, dried milk, fishmeal, Arachis hypogea (groundnut), flour, bran, malt, Linum usitatissimum (flax seed), Medicago sativa (alfalfa seed), Lycopersicum esculantus (tomato seed), Phaseolus vulgaris (pinto beans), Vigna unguiculata (blackeyed cowpeas), Sorghum bicolor (sorghum seed) and many other food products (Lindgren and Vincent 1959; Lindgren et al. 1955).
Geographic distribution
The distribution of khapra beetle extends from Mayanmar (Burma) to West Africa and is limited by the 35° parallel to the north and the equator to the south. It has been introduced by commerce into some areas of similar climatic conditions (Anonymous 1981). The khapra beetle is found in all continents where grain and grain products are stored.
Biology and transmission
The adults are oblong-oval beetles, approximately 1.6 to 3.0 mm long and 0.9 to 1.7 mm wide. Males are brown to black with indistinct reddish brown markings on elytra. Females are slightly larger than males and lighter in color. The head is small and deflexed with a short 11-segmented antenna. The antennae have a club of three to five segments, which fit into a groove in the side of the pronotum. The adults are covered with hairs. The eggs are milky white, turning pale yellowish with age, cylindrical, 0.7 ´ 0.25 mm, one end rounded, the other pointed and bearing spine-like projections. Larvae are uniformly yellowish white, except head and body hairs are brown. As the larvae increase in size, their body color changes to a golden or reddish brown, more body hairs develop, and the tail becomes proportionally shorter. Mature larvae are approximately 6 mm long and 1.5 mm wide. Adult khapra beetles have wings, but apparently do not fly and feed very little. Mated females live from four to seven days, unmated females from 20 to 30 days, and males from seven to12 days. Mating occurs about five days after emergence, and egg laying begins almost immediately at 40°C. Egg laying may begin at one to three days at cooler temperatures, but no eggs are produced at 20°C. Eggs hatch in three to 14 days after the female lays an average of 50 to 90 eggs that are loosely scattered in the host material. Complete development from egg to adult can occur from 26 to 220 days, depending upon temperature. Optimum temperature for development is 35°C. If the temperature falls below 25°C for a period of time or if larvae are very crowded, they may enter diapauses. They can survive temperatures below -8°C. In diapauses, the larvae can molt but are inactive and may remain in this condition for many years (Anonymous 1981).
Detection/indexing methods at ICRISAT
- Dry seed examination using magnifying lens.
Treatment/control
- High concentrations of fumigant (Aluminium phosphide) are maintained during the fumigation period to allow penetration into all cracks and crevices. In an eradication program, both fumigants and surface treatment (chloropyriphos) are used in combination with preventive measures, e.g., good sanitation practices and exclusion.
Procedures followed in case of positive test at ICRISAT
- Rejection and incineration of the infested seed samples.
References and further reading
Anonymous. 1981. Data sheets on quarantine organisms. Trogoderma granarium Everts. European and Mediterranean Plant Protection Organization Bulletin 11 (1) Set 4, List A2, 1-6 pp.
Lindgren DL, Vincent LE. 1959. Biology and control of Trogoderma granarium Everts. Journal of Economic Entomology 52: 312-319.
Lindgren DL, Vincent LE, Krohne HE. 1955. The khapra beetle, Trogoderma granarium Everts. Hilgardia 24: 1-36.
 Khapra Beele (Trogoderma granarium) of sorghum: (A)adults and (B)larvae on the grains (photos:www.agspsrv34.wa.gov.au) |
Best practices for the safe transfer of sorghum germplasm
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
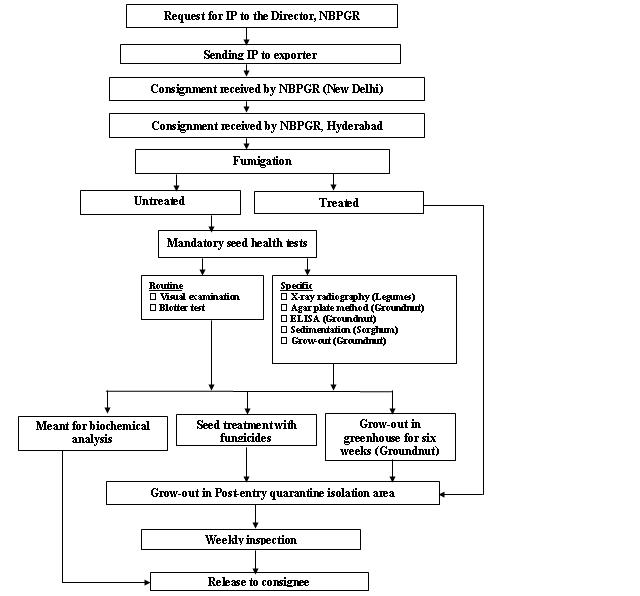
Seed health testing protocol at ICRISAT-PQL for import
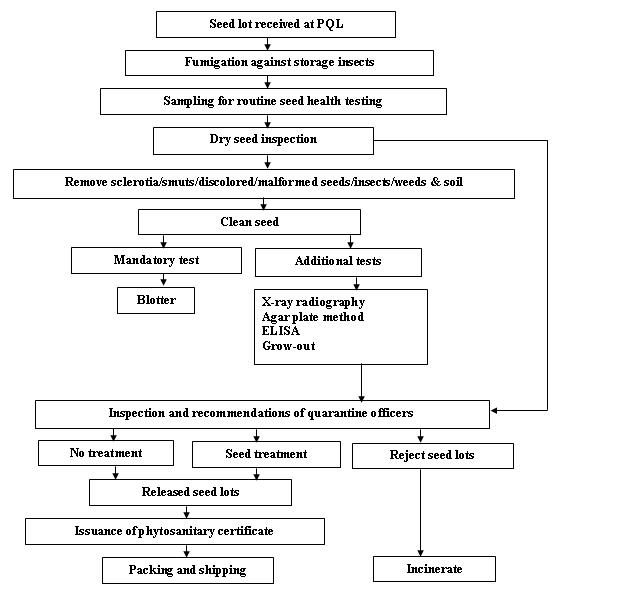
Seed health testing protocol at ICRISAT-PQL for export
References and further reading
Chakrabarty, SK, K. Anitha, AG Girish, B Sarath Babu, RDVJ Prasad Rao, KS Varaprasad, PK Khetarpal and RP Thakur. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin 69. Rajendranagar 500 030, Andra Pradesh, India. National Bureau of Plant Genetic Resources, Pantacharu 502 324, Andra Pradesh, India. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT).