CGKB News and events Management strategies
Import/export of forage grass germplasm
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
CIAT has a plant quarantine agreement with ICA (Instituto Colombiano Agropecuario) establishing guidelines to facilitate germplasm exchange. The agreement permits the transit of seed through customs and quarantine stations according to the level of potential risk of introducing pests and diseases not yet reported in Colombia. ICA has established quarantine procedures to regulate the introduction of plant germplasm and for issuing of phytosanitary certificates.
ILRI follows the current host country regulations of Ethiopia, for the importation of plant materials. Application for import of plant materials stating common name, botanical name and quantity is made to the Ministry of Agriculture and Rural Development, Plant Quarantine Service. A plant importation permit is issued indicating any specific conditions for the species and materials are inspected on entry before being released for use. Export of materials follows a similar process with application to the same office and inspection before a phytosanitory certificate is issued.
Guidelines for safe transfer of forage grass germplasm
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
Technical Guidelines for the Safe Transfer of Germplasm and the
Protection of CGIAR Germplasm Banks
Pathogens of quarantine significance of forage grass (CIAT, ILRI)
|
Viruses
|
|
Digitaria striate rhabdovirus (DSV)
|
|
Elephant grass mosaic virus (EMV)
|
|
Guineagrass mosaic virus (GGMV)
|
|
Johnsongrass mosaic virus (JGMV)
|
|
Maize dwarf mosaic virus (MDMV)
|
|
Maize streak virus (MSV)
|
|
Sugarcane mosaic virus (SCMV)
|
|
Bacteria
|
|
Xanthomonas axonopodispv.phaseoli.
|
|
Xanthomonas campestris pv. graminis (Egli et al.)Dye
|
|
Xanthomonas campestres pv. Phlei
|
|
Fungi
|
|
Ascochyta graminícola (Sacc. [Reported in Andropogon gayanus by Lenné, 1994].
|
|
Ascochyta paspali (H. Sydow) Punith [Reported in Paspalum sp. by Lenné, 1994].
|
|
Botrytis cinerea(Pers. 1794 (Teleomorph. Sclerotinia fuckeliana (de Bary) Fuckel).
|
|
Curvularia spp.
Curvularia cymbopogonis (Dodge) Groves et Skolko – Purple leaf spot
Curvularia penniseti (Mitra) Boedijn Synonym: Acrothecium penniseti Mitra
- Curvularia leaf spot
Curvularia pallescens Boedijn 1933 Synonym: Pseudocochliobolus pallescens
Tsuda & Ueyama
|
|
Drechslera spp.
Drechslera setarie (S. Ito and Kuribay.) Dastur.
D. sacchari (Butler) Subram. and Jain
|
|
Helminthosporium spp. New classification: Exserohilum spp.
|
|
Phoma sorghina (Sacc.) Boerema, Dorenb, and van Kest.
|
|
Pyricularia oryzae Cavara
Synonym: Pyricularia grisea (Cooke) Sacc., anamorph of Magnaporthe grisea (T. T. Hebert) Yaegashi & Udagawa.
|
|
Rhizoctonia solani J.G. Kühn 1858 (Teleomorph Thanatephorus cucumeris (Frank) Donk.)
Synonym: Thanatephorus cucumeris (A.B. Frank) Donk 1956
|
|
Sphacelia sp. (Teleomorph. Claviceps spp.)
Claviceps sp., Claviceps sulcata Langdon, Claviceps cynodontis Langdon, Claviceps pucilla Ces., Claviceps paspali F.L. Stev. and Hall., Claviceps maximens Theiis
|
|
Tilletia aryesii Berk.
|
|
Ustilago kamurensis P. & H. Sydow
|
|
Insects
|
|
Acantoscelides sp.
|
|
Zabrotes spp.
|
|
Nematodes
|
|
Ditylenchus dipsaci (Kühn) Filipjev
|
|
Meloidogyne spp.
|
|
Phytoplasma
|
|
Stunt disease
|
Bacteria - forage grass
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
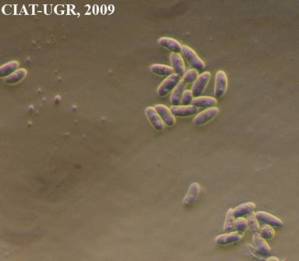
Bacterial Blight of forage grasses
Scientific names
Xanthomonas campestris pv. graminis (Egli et al.) Dye
Xanthomonas campestres pv. phlei
Significance
Affects the value of the forage grass.
Symptoms
Wilting and drying up of the tillers or the whole plants accompanied or not by leaf necrosis; no growth of new shoots after mowing; yellow stripes turn necrotic along the leaf; distorted growth of the leaves out of the sheath or difficulty of cobs (ears, heads) to emerge; young leaves have a pale colour.
In the Lolium multiflorum yellow droplets form inside the hollow of the stem.
Hosts
Arrhenatherum elatius, Dactylis glomerata, Festuca arundinacea, Festuca pratensis, Phleum pretense, Brachiaria spp., Lolium multiflorum, Lolium perenne.
Geographic distribution
Widespread in the tropics and sub-tropics
Biology and transmission
Bacterial wilt settles in the xylem vessels. The disease can only settle through lesions from which it extends to the xylem. It goes counter sap-flow down to the base of the plant and infects the neighbouring tillers.
Considerable damage may occur after long periods of hot and dry weather, while in cool and wet periods hardly any diseased plants are found. The pathogen may be identified by isolation and biochemical tests. Specific antibodies used in immunofluorescence and ELISA had a high degree of sensitivity and specificity against the target bacterium. The two methods were used for screening pure cultures and detecting bacteria directly in plant tissue extracts. Their application revealed the presence of low numbers of bacteria in symptomless plants and a discontinuous distribution within the plant.
Cultures of Xanthomanas campestris pv graminis showed no loss of virulence following freeze-drying and revival.
Inoculation of seeds, and inoculation of plants for seed production, X. campestris pv. graminis was shown to be seed-transmitted. The level of infection in seeds was, however, too low to produce plants with disease symptoms.
The spread inside the crop is caused by the infected cutter bar of forage harvesters which can carry the pathogen for several months.
Detection/indexing method in place at the CGIAR Center
- at CIAT: None
- at ILRI: None
- at ICRISAT: Agar plate method
Treatment/control
- Hot water treatment at 56ºC for 30 minutes
Procedure followed at the centers in case of positive test
- Seeds treated as above to kill bacteria
References and further reading
-
http://www3.interscience.wiley.com/journal/119440154/abstract?CRETRY=1&SRETRY=0
-
http://www3.interscience.wiley.com/journal/119440144/abstract
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. eds. 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Fungi - forage grass
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
Scientific names
Drechslera setarie (S. Ito and Kuribay.) Dastur;
Drechslera sacchari (Butler) Subram. and Jain)
Significance
Several species of Drechslera are important plant pathogens and are associated with symptoms of dark spots on leaves, and root rot of seedlings. Severe forage losses have been recorded in Hawaii and Florida.
Symptoms
Symptoms occur as brown to purple lesions, 1-4 mm in length on leaf blades. Lesions tend to form streaks or eye spots. Leaf spots usually more numerous near the collar area of the leaf blade. Severely affected leaves turn reddish brown, wither and die. Under severe disease pressure, sheath and crown rot may develop resulting in plant death.
Hosts
Cynodon dactylon, Brachiaria spp., Pennisetum purpureum, sugarcane
Geographic distribution
Caribbean, South America, and USA
Biology and transmission
Isolates from sugarcane caused mild symptoms on elephant grass and vice versa.
Detection/indexing method in place at the CGIAR Center
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy. ITSA has approved a detection method of Drechslera oryzae on Oryza sativa (Rice) and is used at GHL [ITSA, 2008].
- at ILRI: None
Treatment/control
- In rice the following fungicides reduced seed infection by Drechslera oryzae: Triphenyltin chloride, Fentin hydroxide, IBP (Kitazin), Zineb and Mancozeb. Two applications of Chlorothalonil (1.5 kg/ha) or Metam-sodium controlled Drechslera oryzae.
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Agarwal VK, Sinclair JB. 1987. Principles of seed pathology. CRC Press, Boca Raton, FL, USA. Volume II Chapter 14 control of seedborne pathogens.
Lenné JM. 1994. Diseases of other pasture grasses. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 169-194. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Weikert-Oliveira RCB. et al. 2002. Genetic variation among pathogens causing "Helminthosporium" diseases of rice, maize and wheat. Fitopatol. bras., vol.27, no.6, p.639-643
References of protocols at EPPO, NAPPO or similar organization
International Seed Testingg Association (ISTA). 2008. International Rules for Seed Testingg Annexe to Chapter 7: Seed Health Testingg Methods. 7-010: Detection of Drechslera oryzae on Oryza sativa (Rice).
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
 Drechslera conidia and culture (photos: URG-Virology Unit, CIAT) |
Scientific names
Sphacelia spp. [anamorphs]
Claviceps spp. [Teleomorphs]
Claviceps africana Freder., Mantle & De Milliano 1991
Claviceps paspali F. Stevens & J.G. Hall 1910
Claviceps phalaridis J. Walker 1957
Cepsiclava phalaridis (J. Walker) J. Walker 2004
Other scientific names
Claviceps purpurea (Fr.) Tul. 1883
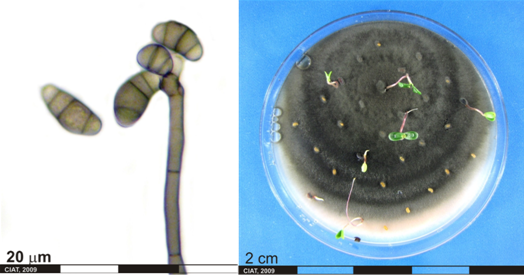
Significance
Phytosanitary importance. May have nil tolerance. Can render grasses toxic and useless as feed or forage. Inflorescence pathogens (eg., Sphacelia) are readily transmitted by seed. This presents risks in moving germplasm between countries, especially from Africa to other continents. Ergot has considerable potential to be damaging to seed production of Brachiaria spp.
Symptoms
The characteristic symptom of this disease is the dark purplish sclerotia (resting body), that develop in place of healthy seed and protrude from the glume. These ergot sclerotia can be up to four times higher than the normal seed.
The appearance of the sclerotia is preceded by the honey dew stage which appears two to three weeks after flowering. The infected florets exude a yellow, sugary sticky fluid. Insects are attracted to feed on this exudate. Affected heads may appear dirty because of the accumulation of dust and pollen on the sticky honey dew. The ergot of phalaris is not typical, as all florets of infected plants show symptoms. The fungus is systemic within the plant and, in a perennial grass such as phalaris, will persist in its host from season to season.
Hosts
Ergot fungi (Claviceps spp.) are parasites on more than 600 grass species, including forage grasses and leading cereals: wheat, rice, barley, sorghum, oats, rye and millet.
Andropogon gayanus, A. tectorum, Brachiaria spp., Cynodon, Hyparrhenia, Panicum maximum, Paspalum commersonii, P. compressum, P. conjugatum, P. dilatatum (paspalum, Dallas grass), P. distichum (P. vaginatum, salt water couch), P. notatum (Bahia grass), P. orbiculare (ditch millet), P. paspalodes (water couch), P. scrobiculatum, P. urvillei (water couch).
Geographic distribution
Worldwide
Biology and transmission
The genus Claviceps includes much specialized fungi which parasitize only the flowers of specific grasses; no other part of the plant is infected. During infection, the ovary is replaced by a specialized fungal structure called a sphacelium that in time becomes another structure called sclerotium (pseudo-seed). The sclerotium resembles a seed grain but is hard, compact mass of fungal tissue with a thin outer layer (rind). Sclerotia of most Claviceps species are one to four times larger than the host seed. Grasses with small seeds, e.g. Agrostis will yield much smaller sclerotia than larger seeded grasses e.g. Lolium.
When the supply of susceptible flowers is depleted, or the crop is harvested, the fungus somehow has to survive a considerable period of time in the absence of the host. The sclerotia provide one possible means of surviving during this period (winter season in Northern Europe). Once favourable environmental conditions reappear at the start of the next crop season, sclerotia may germinate to produce stalked structures that produce spores, which again may infect the flowers of grass plants.
Claviceps spp. carry-over on alternate hosts and as sclerotia on the soil or mixed with seed.
Cool, wet weather in spring that delays pollination, and thus prolongs flowering, also favours germination of the sclerotia. Stands that tiller and flower unevenly or have a high degree of sterility can be severely affected by ergot.
The germinating sclerotia produce spores which are wind-blown and/or rain splashed onto open florets, where they infect the ovary. Within five days a honey dew is produced containing spores which serve as secondary inoculum. These spores are spread to other florets by contact, rain splash and insects.
Detection/indexing method in place at the CGIAR Center
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
Treatment/control
- Thorough seed cleaning by soaking in 30% salt solution and removing any seeds that float
Procedure followed at the centers in case of positive test
- Reject seeds and repeat regeneration
References and further reading
Seed Health General Publication Published by the Center or CGIAR
Miles JW, Maass BL, do Valle CB; with the collaboration of Kumble V. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
 |
 |
|
|
Ergot honeydew and macroconidia [Sphacelia stage] (photos: URG-Virology Unit, CIAT) |
||
Scientific names
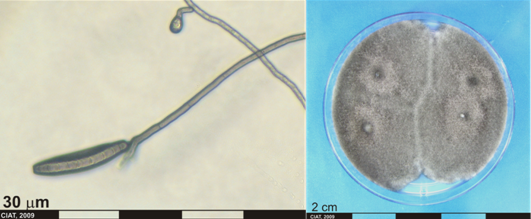
Curvularia spp. [anamorphs]
Curvularia cymbopogonis (Dodge) Groves et Skolko
Curvularia penniseti (Mitra) Boedijn
Pseudocochliobolus pallescens Tsuda & Ueyama [Teleomorph]
Other scientific names
Acrothecium penniseti Mitra - Curvularia pallescens Boedijn 1933.
Significance
Widespread in grasses causing reduced yield
Symptoms
Small yellow-brown spots on leaves expand to oblong lesions. Center of lesions change to brown and margins remain yellow. Lesions are more common on leaf margins.
Hosts
Curvularia cymbopogonis: Andropogon spp.
Curvularia pallescens: Axonopus, Bracharia, Coix, Cymbopogon, Cynodon, Dactyloctenium, Digitaria, Echinochloa, Euchlaena, Imperata, Oryza, Panicum, Paspalum, Pennisetum, Rottboellia, Saccharum, Setaria, Sorghum, Sporobolus, Triticum, Zea
Curvularia penniseti: Pennisetum sp. Sorghum bicolor, Triticum; Isolated from Allium, Dolichos and Richardia
Geographic distribution
C. pallescens: Australia,Barbados, Brunei, Burma, Canada, Cuba, Ghana, Hong Kong, India, Indonesia, Jamaica, Kenya, Malaysia, Malawi, Nepal, Nigeria, Pakistan, Papua New Guinea, Peru, Sierra Leone, Singapore, Solomon Islands, Sri Lanka, Sudan, Tanzania, Venezuela, Zimbabwe.
C. penniseti: Australia, India, Nepal, Nigeria, Pakistan, Zimbabwe
Biology and transmission
Other species of Curvularia can be isolated from pearl millet including: Curcularia lunata (Wakker) Boed. A toxin produced by the pathogen is related to host and cultivar specificity; Curvularia geniculata (Tracy & Earle) Boed.
No information is available to indicate if symptoms caused by other species of Curvularia differ from symptoms caused by Culvularia penniseti.
Curvularia species are frequently isolated from seed.
Detection/indexing method in place at the CGIAR Center
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: Culture on oatmeal agar, isolation and identification under microscope
Treatment/control
- Seedborne and no treatments reported
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration seed process in field.
References and further reading
- www.wvu.edu/~agexten/ipm/pestprog/educate/3forage.PDF
- http://www.nilgs.affrc.go.jp/db/diseases/contents/de21.htm
Prithiviraj B, Singh UP, Manickam M, Srivastava JS, Ray AB. 1997. Antifúngical activity of bergenin, a constituent of Flueggea microcarpa. Plant Pathology 46:224-228.
Lenné JM. 1994. Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Stylosanthes. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 21-42. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Pratt RG. 2006. Johnsongrass, Yellow Foxtail, and Broadleaf Signalgrass as New Hosts for Six Species of Bipolaris, Curvularia, and Exserohilum Pathogenic to Bermudagrass. Plant Disease 90 (4):528.
Roberts JA, Tredway LP. 2008. First Report of Curvularia Blight of Zoysiagrass Caused by Curvularia lunata in the United States. Plant Disease 92 (1):173.
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
 Conidia and culture of Curvularia sp. (photos:CIAT) |
Scientific name
Helminthosporium spp=Exserohilum spp., Bipolaris spp., Drechslera spp.
Significance
Helminthosporium species are causal agents of various diseases that occur in a huge range of plants, including cultivated and wild species.
Symptoms
The genus Helminthosporium includes pathogens whose conidial stages are responsible for serious diseases of rice, corn, grasses, and cereals. In sugarcane is characterized by the formation of eye-shaped lesions, followed by the development of reddish-brown "runners" which extend from the lesion toward the leaf tip.
Hosts
Axonopus spp., Cynodon dactylon, Stylosanthes guianensis, oat, rye, sugar cane, sorghum, Brachiaria decumbens, P. maximum.
Geographic distribution
Cosmopolitan
Biology and transmission
Several members of the genus produce host-specific toxins. These compounds, which are fungal metabolites toxic only to the susceptible host, produce virtually all of the disease symptoms and are critical for pathogenicity of the fungi.
Detection/indexing method in place at the CGIAR Center
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
Treatment/control
- Soak in 0.5% sodium hypochlorite for 10 min
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Artigiani VHF, Bedendo IP. 1995. Patogenicidade de Helminthosporium oryzae a algumas espécies de gramíneas. Sci. agric. (Piracicaba, Braz.), vol.52, no.1
Frederick P, Gary S. 1976. Serinol as an activator of toxin production in attenuated cultures of Helminthosporium sacchari. PNAS 73:4007-4011
Lenné JM. 1994. Diseases of other pasture grasses. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 169-194. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Stylosanthes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 21-42. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/. IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
 Conidia and culture of Helminthosporium sp. (photos:CIAT) |
Leaf Spots, Black Stem Spotting
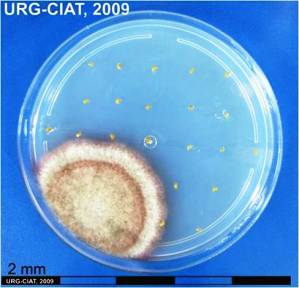
Scientific name
Phoma sorghina (Sacc.) Boerema, Dorenb, and van Kest.
Significance
Widespread causing lodging and yield loss
Symptoms
Causes pre-and postemergent damping-off of several legumes. This pathogen is also commonly associated whit necrotic lesions on leaves of Stylosanthes spp. In tropical South America.
Hosts
Desmodium spp., Brachiaria spp., Centrosema spp., Macroptilium atropurpureum, Stylosanthes spp.
Geographic distribution
Nigeria, South America
Biology and transmission
Symptom expression and pycnidial formation required sustained conditions of high relative humidity, so that recognition of the disease may be difficult under dry conditions.
Detection/indexing method
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: Oatmeal agar culture and identification with microscope
Treatment/control
- Hot water treatment at 500C for 20-30 minutes
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Lenné JM. 1994. Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of other pasture grasses. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 169-194. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Macroptilium atropurpureum. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 77-96. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Stylosanthes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 21-42. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/. IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
 |
 |
|
|
P. sorghina pycnidia and culture (photos: URG-Virology Unit, CIAT) |
||
Scientific names
Pyricularia oryzae Cavara, Pyricularia grisea (Cooke) Sacc., [Anamorphs]
Magnaporthe grisea (T. T. Hebert) Yaegashi & Udagawa. [Teleomorph]
Significance
Pyricularia oryzae is the cause of rice blast, is one of the most important fungal pathogens of rice (Oryza sativa L.) because of its widespread occurrence and destructive nature however this fungi could be found in grass seed.P. grisea is found on grasses as well.
Symptoms
Symptoms are darkened lesions at the panicle neck node and flag leaf collar. Many of the panicles with neck rot were partially filled or blank. The fungus can attack any aerial part of the rice plant, including seeds, in which the fungus may overwinter for several years. There is also latent infection in seedlings by P. oryzae grown under low temperature conditions.
Hosts
Rice (Oryza sativa L.), Lolium perenne, Pennisetum clandestinu, Eleusine indica, Echinochloa colonum
Geographic distribution
Seed transmission of P. oryzae was first reported from Japan and later from other parts of the world.
Biology and transmission
Investigations suggested systemic transmission of the fungus from seeds to seedlings.
Detection/indexing method
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
Treatment/control
- None
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Greer CA, Scardaci SC, Webster RK. 1997. First Report of Rice Blast Caused by Pyricularia grisea in California. Plant Disease 81 (9):1094.
Lenné JM. 1994. Diseases of other pasture grasses. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 169-194. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Manandhar HK. 1996. Rice blast disease: Seed transmission and induced resistance. Ph.D. thesis. The Royal Veterinary and Agricultural University, Copenhagen. Cited in: Manandhar, H. K., H. J. Lyngs Jorgensen, V. Smedegaard-Petersen, and S. B. Mathur. 1998. Seedborne Infection of Rice by Pyricularia oryzae and Its Transmission to Seedlings. Plant Disease 82 (10):1093.
Manandhar HK, Lyngs Jorgensen HJ, Smedegaard-Petersen V, Mathur SB. 1998. Seedborne Infection of Rice by Pyricularia oryzae and Its Transmission to Seedlings. Plant Disease 82 (10):1093.
Radjacommare R, Ramanathan AK, Sible GV, Harish S, Samiyappan R. 2004. Purification and anti-fungal activity of chitinase against Pyricularia grisea in finger millet. World Journal of Microbiology & Biotechnology 20: 251–256.
Rice pathology. In: Centro Internacional de Agricultura Tropical. Integrated Pest and Disease Management in Major Agroecosystems: Project PE-1: Summary Annual Report 2003. CIAT, Cali, CO. p. 199-206.
Wong FP, Gelernter W, Stowell L. 2005. First Report of Pyricularia grisea Causing Gray Leaf Spot on Kikuyugrass (Pennisetum clandestinum) in the United States. Plant Disease 89 (4):433.
Wong FP, de la Cerda KA. 2006. First Report of Pyricularia grisea (Gray Leaf Spot) on Perennial Ryegrass (Lolium perenne) in Nevada. Plant Disease 90 (5):683.
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food andAgriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
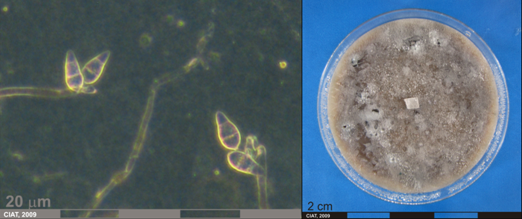 Conidia and culture of Pyricularia oryzae (photos:CIAT) |
Rhizoctonia root rot, Rhizoctonia foliar blight
Scientific names
Rhizoctonia solani Kühn [Anamorph]
Thanatephorus cucumeris ( Frank) Donk [Teleomorph]
Significance
Minor
Symptoms
Both pre-emergent and post-emergence seedling death can occur with this disease. Pre-emergence symptoms are seed decay and are often not visible in the field. Post-emergence symptoms on seedlings will be the appearance of brown to reddish lesions on stems and roots just below the soil line. These reddish brown lesions may become sunken and girdle the stems and kill the plant. Plants may often appear stunted and unhealthy throughout the season or, less commonly, will die. Often the stand will appear uneven because of stunted plants. On older plants, the pathogen causes a reddish brown dry cortical root rot that may extend into the base of the stem. Later in the season, infections at the base of the plant (cortical rot) may result in plants snapping off during high winds. Root rot can greatly reduce nodulation. Foliar symptoms may include yellowing or wilting of leaves.
Damping-off of legume seedlings; web blight resulting in premature defoliation; leaf rust
Foliar blight appears initially as water-soaked patches in the foliage canopy. Profuse growth of fungal mycelium throughout the foliage causes mats of leaves to be stuck together with mycelia strands. Sclerotia are common on blighted leaves. Under prolonged humidity, the patches may extend, causing considerable rotting and death of foliage. Death leaves and petioles are covered and matted together by greyish brown to white fungal mycelium.
Hosts
Brachiaria humidicola, Brachiaria dictyoneura, Brachiaria brizantha
Zea mays
Geographic distribution
USA
This disease has been recorded in Central and South America, Florida (USA), Malaysia, Papua New Guinea, Solomon Islands, Zambia and globally throughout the tropics.
Biology and transmission
Rhizoctonia solani can be found in most soils and survives as sclerotia (very resistant fungal survival structures) in soil.
Rhizoctonia root rot is usually common in warm, moist sandy soils. Stresses in plants which have been observed to favor disease development include herbicide injury, soil insect damage, hail, sandblasting and soybean cyst nematode feeding.
Damage from Rhizoctonia is commonly observed in areas when there is a long history of soybean production with close rotations or during weather conditions not favourable for seed germination and rapid growth of seedlings.
Initiation and development of foliar blight from foci at the beginning on the wet season are favored by high relative humidity, frequent prolonged rain and moderately high temperatures. In Central and South America, foliar blight is most severe in regions with greater than 1500 mm mean annual precipitation. The fungus probably spreads from perennial plants in the pasture and plants debris in the soil. The pathogen complex is primarily soil-borne. Sclerotia readily survive in soil for several years and are disseminated by wind, rain and animals.
Detection/indexing method
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
- at ICARDA: PDA
Treatment/control
- Fungicides can be used
Procedure followed at the centers in case of positive test
- Crop rotation, fungucid seed treatments
- Reject accession and new regeneration started in field.
References and further reading
- http://pdc.unl.edu/agriculturecrops/soybean/rhizoctonia
- http://www.fao.org/ag/AGP/AGPC/doc/Publicat/Gutt-shel/x5556e0s.htm
Lenné JM. 1994. Diseases of Aeschynomene. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 97-107. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI: Oxon, GB.
Lenné JM. 1994. Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Macroptilium atropurpureum. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 77-96. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
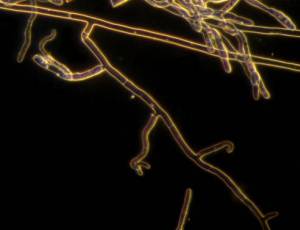 |
 |
|
|
R. solani hyphae and culture (photos: URG-Virology Unit, CIAT) |
||
Scientific names
Tiletia aryesii Berk.
Significance
Smut substantially reduces seed production in tropical America. Although seed production may be greatly reduced, plant vigor does not appear to suffer.
Symptoms
Spikelets of affected inflorescences are open, swollen and filled with semiagglutinate, greyish spore masses, which are released in a grey could when inflorescence are shaken.
Hosts
Panicum maximum, Panicum spp., Setaria spp.
Geographic distribution
Africa: Cameroon, Congo, Ethiopia, Ghana, Ivory Coast, Kenya, Madagascar, Malawi, Mali, Mauritius, Mozambique, Nigeria, Sierra Leone, South Africa, Sudan, Tanzania, Togo, Uganda, Zaire, Zambia, Zimbabwe; Asia: Sri Lanka; Central America: Costa Rica; South America: Brazil, Colombia
Biology and transmission
As spores are wind-borne, infection of opened flowers readily occurs. Naturalized plants around pastures are a major source of infection for seed crops. Smut is also seed-borne.
Detection/indexing method
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
Treatment/control
- Many seeds are destroyed by smut, but if any are harvested they can be treated with Copper Oxide, Carboxin and Benomyl or hot water to kill spores.
Procedure followed at the centers in case of positive test
Reject accession and new regeneration started in field.
References and further reading
Lenné JM. 1994. Diseases of other pasture grasses. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 169-194. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Mordue JEM, IMI Descriptions of Fungi and Bacteria Tilletia ayresii. CABI Bioscience, Bakeham Lane, Egham, Surrey, TW20 9TY, UK.
Scientific names
Ustilago kamerunensis P. & H. Sydow
Significance
Major pest and disease threat to Napier grass.
Affects adversely the small-scale diary industry.
Symptoms
Napier grass, turning vigorous, impenetrable clumps of valuable livestock feed into thin, shrivelled stems.
Presence of fungal spores in the inflorescence.
Hosts
Pennisetum purpureum
Geographic distribution
Africa: Kenya
Biology and transmission
The main means of spread is through transport of infected planting material.
Smut disease causes serious loss of biomass of fodder crops.
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: Potato dextrose media, isolation and identification
Treatment/control
- Napier grass rarely produces sseeds and the mycelia remain in the cuttings or in the embryo of nay seeds. If seeds are harvested, they can be treated with systematic fungicides or hot water.
Procedure followed at the centers in case of positive test
- Rogue and burn all infected plant tissue.
References and further reading
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
More Articles...
- Nematodes - forage grass
- Best practices for safe transfer of forage grass germplasm
- Viruses - forage grass
- Insects - forage grass
- Weeds (Forage grasses)
- Phytoplasma - forage grass
- Safe transfer of maize germplasm
- Import/export of maize germplasm
- Guidelines for the safe transfer of maize germplasm
- Bacteria - maize
Subcategories
-
main
- Article Count:
- 11
-
Stog
- Article Count:
- 2
-
Stog-rice
- Article Count:
- 7
-
Stog-sorghum
- Article Count:
- 11
-
Stog-common-bean
- Article Count:
- 10
-
stog-forage-legume
- Article Count:
- 10
-
stog-forage-grass
- Article Count:
- 11
-
stog-maize
- Article Count:
- 9
-
stog-chickpea
- Article Count:
- 10
-
stog-millets
- Article Count:
- 12
-
stog-barley
- Article Count:
- 10
-
stog-groundnut
- Article Count:
- 9
-
stog-pigeon-pea
- Article Count:
- 8
-
stog-wheat
- Article Count:
- 10
-
stog-lentil
- Article Count:
- 9
-
stog-cowpea
- Article Count:
- 10
-
stog-faba-bean
- Article Count:
- 9
-
risk management
- Article Count:
- 4
-
decision support tool
- Article Count:
- 3
-
stog-clonal
- Article Count:
- 23
-
developing strategies
- Article Count:
- 4




