CGKB News and events Management strategies
Import/export of common bean germplasm
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck).
CIAT has a plant quarantine agreement with ICA (Instituto Colombiano Agropecuario) establishing guidelines to facilitate germplasm exchange. The agreement permits the transit of seed through customs and quarantine stations, according to the level of potential risk of introducing pests and diseases not yet reported in Colombia. ICA has established quarantine procedures to regulate the introduction of plant germplasm and for issuing of Phytosanitary Certificates.
Guidelines for safe transfer of common bean germplasm
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck).
Technical Guidelines for the Safe Transfer of Germplasm and the
Protection of CGIAR Germplasm Banks
Pathogens of quarantine significance of common bean tested by the Germplasm Health Laboratory of CIAT
|
Viruses |
|
Peanut mottle virus (PeMOV) |
|
Bean common mosaic virus (BCMV) |
|
Bean southern mosaic virus (BSMV) |
|
Bacteria |
|
Xanthomonas axonopodis pv. phaseoli |
|
Pseudomonas syringae pv. phaseolicola |
|
Curtobacterium flaccumfasciens |
|
Pseudomonas fluorescens Biotipo II |
|
Fungi |
|
Alternaria spp. |
|
Ascochyta phaseolorum Sacc. Current name: Phoma exigua var. exigua Sacc. 1879 |
|
Botrytis cinerea Pers. (Teleomorph. Sclerotinia fuckeliana (de Bary) Fuckel) |
|
Cercospora canescens Ellis & G. Martin |
|
Colletotrichum gloeosporioides (Penz.) Penz. And Sacc. |
|
Colletotrichum lindemuthianum (anamorph.), Gloromella cingulata (teleomorph.) |
|
Colletotrichum truncatum (Schwein.) Andrus |
|
Fusarium oxysporum Schltdl. Y F. solani f. sp. solani |
|
Macrophomina phaseolina (Tassi) Goid |
|
Phoma exigua Desmaz. var. diversispora (Bubak) Boerema |
|
Phomopsis subcircinata (anamorph.), Diaporthe phaseolorum (teleomorph) |
|
Phaeoisariopsis griseola (Sacc.) Ferraris |
|
Rhizoctonia solani Kühn (Teleomorph Thanatephorus cucumeris (frank) Donk.) |
|
Sclerotinia sclerotiorum |
|
Sclerotium rolfsii Sacc. (Teleomorph. Corticium rolfsii Curzi) |
|
Insects |
|
Acantoscelides sp. |
|
Zabrotes spp. |
|
Nematodes |
|
Meloidogyne spp. |
|
Pratylenchus spp. |
Bacteria - common bean
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck).
|
Contents: |
Common bacterial blight, fuscus blight
Scientific name
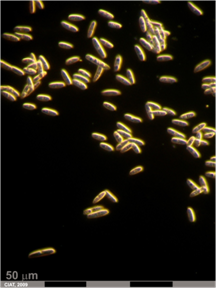
Xanthomonas axonopodis pv. phaseoli (Smith) Vauterin, et al.
Other scientific names
Xanthomonas campestris pv. phaseoli (Smith) Dye;
Xanthomonas phaseoli var. fuscans (Burkholder) Starr & Burkholder
Significance
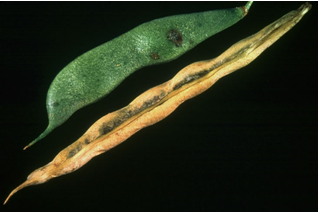
It is not always possible to separate the losses caused by common blight and those due to halo blight, Pseudomonas syringae pv. phaseolicola, since they frequently occur together in the same field and even on the same plant. Moreover, the severity of blight varies from year to year depending on weather conditions. In general, Xanthomonas axonopodis pv. phaseoli causes the most severe disease under fairly high temperature conditions (25-35°C), and also requires high rainfall and humidity. Yield losses estimated at 22% and 45% have been obtained by natural and artificial infections, respectively, in Colombia. Economic surveys, based upon field observations in the same region, estimated yield losses of 13%.
Symptoms
Induced symptoms on leaves, stems, pods, and seeds: Leaf symptoms initially appear as water-soaked spots which enlarge and frequently coalesce with adjancent lesion. Infected tissues appear flaccid and lesions are often encircled by narrow area of lemon-yellow tissue. Necrosis then develops and may become extensive enough to cause defoliation or stem girdle. Infected seeds are sometimes wrinkled and the hilum may be discoloured. Symptoms are similar to halo-blight of bean. Symptoms on the seed: If the infection occurred when the pods were young, the seed may rot or be wrinkled and shrivelled. If the bacteria enter by way of the funiculus, only the hilum may be discoloured, but this is difficult to detect on dark-seeded varieties. Strains producing the brown pigment (so-called fuscans strains) give more conspicuous seed discoloration.
Hosts
The principal host is Phaseolus vulgaris and Phaseolus lunatus but other legume species are naturally infected, including P. lunatus, Vigna aconitifolia, V. radiata, and Vigna umbellate. Lablab purpureus and Mucuna deeringiana are possibly natural hosts. P. coccineus, P. acutifolius and Lupinus polyphyllus are hosts only by artificial inoculation (Bradbury, 1986). Macroptilium lathyroides.
Geographic distribution
Cosmpolitan
Biology and transmission
The bacterium enters the leaves via stomata or wounds, and subsequently invades the intercellular spaces, causing a gradual dissolution of the middle lamella. The stem is entered in three ways: via the stomata of the hypocotyl and epicotyl; through the vascular system of the leaf; or from infected cotyledons. The seed is penetrated via the vascular system of the pedicel and funiculus. The micropyle also serves as a point of entry into the seed. Direct penetration of seed has not been observed. The pathogen either remains in the seedcoat or passes to the cotyledons when the seed germinates, resulting in infection of the young plants. The bacterium can remain viable for several years beneath the seedcoat. The disease is severe under conditions of high rainfall and humidity, with maximum development around 28°C. Dissemination in the field occurs in conditions of wind-driven rain, and via insects.
Detection/indexing method at CIAT
- Agar plate dilution technique. MXP (Claflin et al, 1987) and YDCA semiselective culture medium and serology with commercial kit.
Treatment/control
Control methods include: planting disease-free seed, avoiding disease by suitable choice of planting date, crop rotation (an 85% reduction of attack was obtained by alternating bean and maize crops in Romania), sprays and dusts (e.g. with copper compounds or streptomycin), and resistant cultivars (Leakey, 1973). Numerous sources of tolerance have been identified, but breeding is complicated by the fact that different genetic systems control the reactions in pods and leaves (Coyne and Schuster, 1974).
Procedure followed in case of positive test
- Reject the accession and initiate new seed regeneration process in the field.
References and further reading
Afanador L, Victoria JI. 1981. Specific of ELSIA to Xanthomonas campestris pv. phaseoli identification. In: Lozano JC. Proccedings of the 5th International Conference on Plant Pathogenic Bacteria at CIAT, Cali, Colombia.
Claflin LE, Vidaver AK, Sasser M. 1987. MXP, a semi-selective medium for Xanthomonas campestris pv. phaseoli. Phytopathology 77(5):730-734.
EPPO/CABI. 1996. Xanthomonas axonopodis pv. phaseoli. In: Smith IM, McNamara DG, Scott PR, Holderness M, editors. Quarantine pests for Europe. 2nd edition. CAB INTERNATIONAL, Wallingford, UK. OEPP/EPPO Data sheets on quarantine organisms. Prepared by CABI and EPPO for the EU under Contract 90/399003.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome / International Board for Plant Genetic Resources.
International Rules for Seed Testing (ISTA), Bassersdorf, Swizerland. 2007. Dectection of Xanthomonas axonopodis pv. phaseoli var. fuscans on Phaseolus vulgaris. Annex to Chapter 7. Seed Health Testing Methods.
Saettler AW, Perry SK. 1972. Seed transmitted bacterial diseases in Michigan navy (pea) beans, Phaseolus vulgaris. Plant Dis. Reptr 56:378-381.
 |
 |
|
|
Common bacterial blight (source: http://photos.eppo.org/index.php/image/975-xantph-01/hits/88-xanthomonas-axonopodis-pv-phaseoli-xantph) |
||
Scientific name
Pseudomonas syringae pv. phaseolicola
Other scientific names
Pseudomonas savastanoi pv. phaseolicola
Significance
It is a legume pathogen of worldwide importance. The bacterium has tremendous disease potential.
Symptoms
Symptoms first appear as dark, water-soaked leaf spots, up to 3mm in diameter and sometimes surrounded by a broad lemon-coloured halo. No lesions have been observed on stems or leaf stalks. When these pathogenic bacteria enter the leaves through wounds or natural openings like stomates they multiply rapidly and induce the formation of lesions. The lesions, which are watersoaked at first, soon become brown and dry and are usually surrounded by a yellow halo. When many lesions grow together, large areas of dead leaf tissue may develop. The halo is actually caused by a toxin that is produced by the bacteria within the lesion. As the toxin diffuses out into the leaf tissue it causes a breakdown of chlorophyll and creates the halo. When toxin from infected leaves is transmitted to the growing point of the shoot, the new leaves that develop are often stunted and chlorotic. Developing seed may rot or become shivelled and discoloured.
Hosts
Cajanus cajan, Lablab purpureus, Macroptilium spp., M. atropurpureum, Phaseolus coccineus, P. lunatus, P.vulgaris, Pueraria spp., Vigna angularis, V. radiata, Neonotonia wightii, Centrosema pubescens.
Geographic distribution
The bacteria have a worldwide distribution. They are found in regions of latin American which have moderate temperatures such as the southern Andes zones of Peru and Colombia, in southern Chile and Brazil, and in the Great Lakes Region of Africa, eastern Africa, including Malawi, Kenya, Zambia, and occasionally Uganda.
Biology and transmission
Cells of the pathogen are single, straight rods and move by multitrichous polar flagellar. They are Gram-negative and strictly aerobic. The optimal growth temperature is 20-23oC and on agar the bacterium produces white to cream coloured colonies that exhibit a bluish tinge and often a green fluorescent pigment. P. syringae pv. phaseolicola survives in infected seeds and plant residues on the soil surface. The organism survives in these habitats until environmental conditions are favourable for infection. Isolates of the pathovar are categorized into three races on the basis of the reactions of a range of differential bean cultivars. Seed transmission is higher when infection occurs earlier in plant development. P. syringae pv. phaseolicola multiplies rapidly on or near the surface of foliage with or without lesion in the presence of des. It is disseminated between leaves and plants by water splash and wind during periods of rainfall.
Detection/indexing method at CIAT
- Agar plate dilution technique. King B semiselective culture medium and serology with commercial kit.
Treatment/control
The best method of control is the use of disease-free seed produced under conditions unfavorable to the organism. Contaminated seed also can be treated with chemicals or antibiotics to destroy bacteria present on the surface. Chemical treatment is seldom effective against internally borne bacteria. Halo blight has been controlled chemically with Bordeaux mixture, copper oxychloride, copper sulfate, copper oxide, streptomycin sulfate, and dihydrostreptomycin sulfate.
Procedure followed in case of positive test
- Reject the accession and initiate new seed regeneration process in the field.
References and further reading
Buruchara RA, Pastor–Corrales MA. 1981. Variation, virulence of Pseudomonas syringae pv. phaseolicola on beans in Colombia. In Lozano JC, editor. Proceedings of Fifth International Conference on Plant Pathogenic Bacteria, August 16-23, 1981, at CIAT, Cali Colombia, pp. 341-351.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome / International Board for Plant Genetic Resources, Rome.
Legard DE, Schwartz HF. 1987. Sources and management of Pseudomonas syringae pv. phaseolicola and Pseudomonas syringae pv. syringae epiphytes on dry beans in Colorado. Phytopathology 77(11):1503-1509.
Taylor JD. 1970. The quantitative estimation of the infection of bean seed with Pseudomonas phaseolicola (Burkh.) Dowson. Ann appl. Biol. 66:29-36.
Taylor JD, Teverson DM, Allen DJ, Pastor-Corrales MA. 1996. Identification and origin of races of Pseudomonas syringae pv. phaseolicola from Africa and other bean growing areas. Plant Pathology 45:469-478.
Schwartz HF. 1989. Halo blight In: Schwartz HF, Pastor-Corrales, MA. Bean Production Problems in the tropics. CIAT. Cali, Colombia, pp 285-302.
 |
 |
|
|
Halo Blight (photo: Howard F. Schwartz, Colorado State University, Bugwood.org) |
||
Bacterial blight, Bacterial Pod Rot
Scientific name
Pseudomonas fluorescens Biotype II
Significance
Pastures of this legume are often severely affected in Colombia, with up to 50% decrease in dry-matter yield. Seed production is also reduced. Under humid conditions, pods and seed rot rapidly, reducing seed production. Seed infection can be as high as 95%.
Symptoms
The disease is characterizates by water-soaked lesions on the young plant, especially young leaves, petioles and terminals, progressing to blight, necrosis, dieback and defoliation. Necrotic spots develop on older leaves and flowering and seed production may be severely reduced.
Hosts
Centrosema sp. (C. acutifolium, C. pubescens, C. brasilianum, C. macrocarpum, C. schiedeanum and C. virginianum), Allium, Brassica, Phaseolus sp., Solanum spp., Leucaena leucocephala, Leucaena esculenta, L. pulverulenta, L. diversifolia and L. shannoni.
Geographic distribution
Belize, Brazil, Colombia, Guatemala, Mexico and Panama.
Biology and transmission
Although the species is normally considered to be a saprophyte, Biotipe II causes root rots and leaf blights on a range of hosts, including legumes. It is distinguished from other fluorescent pseudomonas by the following characteristic: more than one polar flagellum, no poyocyanin or carotenoid pigments produced, no growth at 41°C, gelatin hydrolysed but not starch, and utilization of a wide range of carbon sources. Disease is favoured by high relative humidity and moderately high temperatures. The bacterium can survive unfavourable periods on affected plants and soil for as long as six weeks. The bacterium is seed-borne and levels of infections as high as 32% have been found in seed lots of C. acutifolium.
Detection/indexing method at CIAT
- Agar plate dilution technique. King B semiselective culture medium.
Treatment/Control
Not noted
Procedure followed in case of positive test
- Reject the accession and initiate new seed regeneration process in the field.
References and further reading
Arias B, Lenne JM. 1988. Sistemas de produccion de Pasturas Tropicales de semillas de Centrosema acutifolium y efecto de bacterioocidas en la incidencia de Pseudomonas fluorescens Biotipo II. Pasturas Tropicales Vol. 10:11-18.
Bradbury JF. 1986. Guide to plant pathogenic Bacteria, CAB Interantional, Wallingford, UK.
Guevara Gómez CL, Lenné JM, Torres GC. 1983. Etiology of dieback of Centrosema spp. and effect of the pathogen on yield and quality during the period of establishment of the legume. Phytopathology 73:122.
Lenne JM, Torres GC, Victoria JI. 1981. Bacterial leaf spot and dieback of Centrosema spp. Proceedings of the Fifth International Conference on Plant Pathogenic Bacteria. Cali, Colombia, pp. 35-38.
Scientific name
Curtobacterium flaccumfasciens pv. flaccumfaciens (Hedges) Collins & Jones
Significance
Following the first report of its occurrence in 1920, C. flaccumfaciens pv. flaccumfaciens became one of the most important bacterial diseases of beans in the USA, resulting in almost total crop losses in some years. More recently, however, it has become very much less important and has indeed not been reported on beans since the early 1970s (Hall, 1991). In soyabeans the disease was not reported in the USA until 1975 and is of rather minor importance (Sinclair & Backman, 1989). In the EPPO region, it is important on beans in Turkey, but causes only minor losses in other countries.
Symptoms
Seedlings are stunted, wilted and usually die. In some plants the affected parts are dull green in colour and sometimes the stems break. Infected pods show discolored sutures and may show yellowish areas. Foliar interveinal choruses and necrosis. When the lower stem and root are cut longitudinally and observed, the vascular system is often discolored brown to black. The younger the plant becomes infected, the more severe the damage to the plant. Seedlings are frequently severely stunted or killed. If plants survive to maturity, seeds may show yellow or purple discoloration.
Hosts
Lablab purpureus, Phaseolus coccineus, Phaseolus lunatus, Phaseolus vulgaris, Vigna angularis, Vigna unguiculata, Zornia spp. and possibly Glycine max, Lupinus polyphyllus, Vigna cylindrica, V. sesquipedalis, Dolichos labllab, Phaseolus radiatus, P. lathyroides, P. calcaratus, and P. acutifolius. All members of Leguminosae.
Geographic distribution
Cosmopolitan.
Biology and transmission
Curtobacterium flaccumfasciens pv. flaccumfaciens exhibits the following characteristics: cells are slightly curved rods with some straight and some wedge-shaped. The bacterium is gram-positive, strictly aerobic, and motile by one, or rarely two or three, polar or subpolar flagella, seed is transmitted externally or internally in Phaseolus vulgaris and possibly in Glycine max. The bacterium can be transmitted both within and on the seed; it is very resistant to drying and can remain viable for up to 24 years in seed stored in the laboratory. In the field it has been known to survive in soil for at least two winters between bean crops rotated with wheat. There are no reports of vectors, but the nematode Meloidogyne incognita may assist entry by providing wounds. C. flaccumfaciens pv. flaccumfaciens can infect in the absence of rain; it has not been observed to enter via stomata. Once within the plant, the bacterium colonizes the vascular tissue.
Detection/indexing method at CIAT
- Agar plate dilution technique. NBY (Nutrient Broth Yeast extract) semiselective culture medium and serology with commercial kit.
Treatment/control
Control may be effected by using disease-free seed and crop rotations. Seed grown in dry climates is usually free from infection and is, therefore, recommended for distribution. Some tolerant, but no immune, cultivars have been found.
Procedure followed in case of positive test
- Reject the accession and initiate new seed regeneration process in the field.
References of protocols at EPPO
OEPP/EPPO (1982) Data sheets on quarantine organisms No. 48, Corynebacterium flaccumfaciens. Bulletin OEPP/EPPO Bulletin 12(1).
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Hedges F. 1926. Bacterial wilt of beans (Bacterium flaccumfaciens Hedges), including comparisons with Bacterium phaseoli. Phytopathology 16:1-22.
Torres GC, Lenne JM, Victoria JI. Bacterial wilt of Zornia spp. caused by Corynebacterium flaccumfaciens. Conference paper Proceedings of the Fifth International Conference on Plant Pathogenic Bacteria. 1982 pp. 74-79.
 |
 |
|
|
Bacterial wilt of beans (images courtesy of Howard F. Schwartz and EEPO gallery) |
||
Fungi - common bean
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck).
Scientific names
Colletotrichum lindemuthianum, C. gloeosporioides and C. truncatum
Significance
The main pathogen of disease is Colletotrichum lindemuthianum, although C. gloeosporiodies and C. truncatum also can be present in seed infected. Anthracnose is probably the most important disease of beans throughout the world; this disease has caused severe damage on susceptible bean cultivars or when badly contaminated seed is planted and favorable conditions prevail during the growing seasons [Pastor-Corrales & Tu, 1989].
Symptoms
Symptoms of anthracnose can appear on any plant part, although initial symptoms may appear on cotyledonary leaves as small, dark brown to black lesions. The infected tissues manifest minute rust-colored specks, it gradually enlarge longitudinally and form sunken lesions or eye spots that reach the hypocotyl of the young seedling, causing it to rot off [Pastor-Corrales & Tu, 1989].
Lesions may first develop on leaf petioles and the lower surface of leaves and leaf veins as small, angular, brick-red to purple spots which become dark brown. Later the lesions may also appear on veinlets on the upper surface of leaves. Sporulation can occur in lesion on the petiole and larger leaf veins. Pod infection appear as flesh to rust-colored lesions. The lesions developed into sunken cankers (1-10 mm in diameter) that are delimited by a slightly raised black ring and surrounded by a reddish brown border [Pastor-Corrales & Tu, 1989].
The conidia that can appear as a gelatinous mass in the lesion center, with age, becoming gray-brown or black granulations. If severely infected young pods shrivel and dry up. The fungus can invade the pod, and the mycelia and conidia infect the cotyledon or seed coat of the developing seed. Infected seed are often discolored and may contain dark brown to black cankers [Pastor-Corrales & Tu, 1989].
 Anthracnose (photo: CIAT) |
Hosts
Colletotrichum lindemuthianum is primarily a pathogen of the common bean Phaseolus vulgaris L., but, it can infect related species and varieties such as P. vulgaris var. arborigineus (Burk.) Baudet; P. acutifolius A. Gray var. acutifolius; P. coccineus L.; P. lunatus L.; P. lunatus var. macrocarpus; Vigna mungo (L.) Hepper; V. radiata (L.) Wilczer var. radiata; V. ungiculata (L.) Walpers ssp. ungiculata; Lablab purpureus (L.) Sweet;Vicia fabia L [Pastor-Corrales & Tu, 1989].
Geographic distribution
Bean anthracnose has worldwide distribution. However it causes greater losses in temperature and subtropical zones than in the tropics. Its distribution is in North, Central and South America, Europe, Africa Australia and Asia [Pastor-Corrales & Tu, 1989].
Biology and transmission
The perfect stage of Colletotrichum lindemuthianum (Sacc. et Mang) Scrib. is Gloromella cingulata f.sp. phaseoli [Neergaard, 1977], but is rarely found in culture or in nature. Thus, the name of the imperfect stage is commonly used [Pastor-Corrales & Tu, 1989].
Conidia are borne in an acervulus which may be present on pods, leaves, stems and branches. They may be intra and subepidermal cell walls of the hosts. Conidia are unicellular, hyaline, cylindrical with both ends obtuse or with a narrow and truncate base. Conidia are uninucleate, and usually have a clear vacuole-like body near the center [Pastor-Corrales & Tu, 1989].
During germination of the infected seeds, the pathogen is transferred from seed coat, by colonizing the cotyledons, radicle and plumule of the emerging seeds. Anthracnose inoculum in the field transferred to the young pods, consequently enabled the pathogen to grow through the entire pod surface, showing sunken anthracnose lesions and infected the seed coats of the newly formed seed [Yesuf & Somsiri, 2005].
Has been reported that a different species of Colletotrichum was isolated from bean plants showing anthracnose symptoms in Brazil and Colombia. Inoculate seedlings of different beans with isolated of this pathogen, showed anthracnose symptoms, identified the fungus as C. dematium f. trumcata (Schw.) von Arx., the soybean anthracnose pathogen [Pastor-Corrales & Tu, 1989].
Colletotrichum lindemuthianum can overwinter either in seed or infected crop residues. It can survive for at least two years in seed. Moisture is an important factor that influences the survival of the fungus. C. lindemuthianum survives as dormant mycelium within the seed coat, sometimes even in cells of cotyledons, as spores between cotyledons, or elsewhere in the seed. Temperature and humidity conditions are important for infection and expression of symptoms [Pastor-Corrales & Tu, 1989].
Detection/indexing method at CIAT
- Blotter test method [Kameswara et al., 2006; ISTA, 2008] and mycological characterization throughout stereoscopy and optical microscopy.
Treatment/control
Control by cultural practices includes the sowing en regions with high temperature and low humidity conditions that are unfavorable for infection by and survival of anthracnose fungus. Use of pathogen-free seed considerably reduces losses. Crop rotations of two to three years are recommended because the pathogen can survive in infected crop debris for two or more years; moreover infected plant debris must be removed from the field soon after harvest. It is important to restrict the activity and movement of men and agricultural implements in a field when the foliage is wet from rain or dew [Pastor-Corrales & Tu, 1989].
Chemical treatments have been used for seed treatment. Seed-coat infestations are controlled effectively with Ferbam, Ziram and Ceresan (0.5 g/100 g of seed). However, internal seed contamination is not reduced. The fungicides Benomyl or Thiophanate Methy (5,2 g/kg of seed) where used, better than 95% control was achieved. Preventive spraying of foliage at flower initiation, late flowering and pod-filling with protective systemic fungicides; Maneb and Zineb at 3.5 g/l, Benomyl at 0.55 g/l, Caftacol at 3.5 kg/ha, carbendazim at 0.5 kg/ha, and Fentin hydroxine at 1.2 g/l have been used to control anthracnose. Combination and rotation of these fungicides is ideal [Pastor-Corrales & Tu, 1989]. Seed treatment with Benomyl and Orthocide give best control [Frison et al., 1990].
Seed treatment with the three commercial formulations of Bacillus subtilis and one of Pseudomonas chlororaphis and Curtobacterium sp. had a significant disease-suppressive effect resulting from seed-borne infections by C. lindemuthianum [Tinivella et al., 2009].
Procedure followed in case of positive test
Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References of protocols at ISTA
International Rules for Seed Testing (ISTA). 2008. Annex to Chapter 7: Seed Health Methods: 7-006-3: Detection of Colletotrichum lindemuthianum on Phaseolus vulgaris (Bean)
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Neergaard P. 1977. Seed pathology. John Wiley, New York, NY, USA.
Pastor-Corrales MA, Tu JC. 1989. Anthracnose. 2. Ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 77-104.
Tinivella F, Hirata LM, Celan MA, Wright SAI, Amein T, Schmitt A, Koch E, Wolf JM, Groot SPC, Stephan D, Garibaldi A, Gullino ML. 2009. Control of seed-borne pathogens on legumes by microbial and other alternative seed treatments. Eur J Plant Pathol 123:139–151.
Yesuf M, Sangchote S. 2005. Seed transmission and epidemics of Colletotrichum lindemuthianum in the major bean growing areas of Ethiopia. Kasetsart Journal. Natural Science 39(1):34-45.
Scientific name
Phaeoisariopsis griseola (Sacc.) Ferraris
Other scientific name
Isariopsis griseola Sacc.
Significance
This is a serious disease of bean which has occurred in such tropical and tropical countries. Yield losses can be severe and have reached 50% in U.S., 40-80% in Colombia, 45% in Brazil, and 80% in Mexico [Correa et al., 1989].
Symptoms
Angular leaf spots symptoms occur on all aerial parts of the plant. Lesions are most common on leaves and usually appear within six days after inoculation. They may appear on primary leaves, but usually do not become prevalent on later foliage until late flowering or early pod set. Lesions initially are grey or brown, may be surrounded by a chlorotic halo, and have indefinite margins. They become necrotic and well defined with the typical angular shape by nine days after infection [Correa et al., 1989].
Lesion then may increase in size, coalesce, and cause partial necrosis and yellowing of leaves which then fall off prematurely. On primary leaves, lesions are usually rounded, larger than those found on trifoliate leaves, and may develop concentric rings within themselves. Lesion size is inversely related to lesion number per leaf or leaflet [Correa et al., 1989].
Lesions appear on pods as oval to circular spots with reddish brown centers that are sometimes surrounded by darker colored borders. Infected pods bear poorly developed or entirely shriveled seeds [Correa et al., 1989]. In seeds, symptoms can appear as seed discoloration [Neergaard, 1977].
 Angular leaf spot (photo: Biolib, 2009.) |
Hosts
The fungus has a host range which includes: Phaseolus vulgaris L.; P. lunatus L, P. coccineus L; P. acutifolius A. Gray var. Acutifolius; Vigna mungo (L.) Hepper;I V.angularis (Willd.) Ohwi et Ohashi; V. umbellata (Thunb.) Ohwi et Ohashi; V. ungiculata (L.) Walpers ssp. ungiculata; Pisum sativum L [Correa et al., 1989], Desmodium cephalotus, D. gangeticum, D. pulchellum, Dolichos lablab [Frison et al., 1990].
Geographic distribution
Cosmopolitan
Biology and transmission
The infection cycle in angular leaf spot can be decomposed into five groups of processes Lesion establishment; Lesion extension; Defoliation of infected host leaves bearing lesions; Sporulation on infectious sites; Spore dispersal, (spore liberation and spore deposition) [Allorent & Savary, 2005].
The pathogen infects leaf tissue by entering stromata and advancing intracellularly in the mesophyll and palisade parenchyma. Nine days after infection, the fungus develops intracellularly throughout necrotic lesions. Stromata develop in the substromatal cavity and sporulation may then occur during periods of continuous moisture. Although stromata formation, accompanied by spore release and dissemination, and disease development also can proceed under relatively dry conditions and fluctuating weather conditions (temperature, relative humidity and sunlight) usually favor disease development under field conditions [Correa et al., 1989].
Infection and disease development can occur in a range between 16-28° C, with an optimum of 24° C. Severe disease symptoms in the field by ALS are not usually observed after flowering or as plants approach maturity [Correa et al., 1989].
Contaminated seed constitutes one source of primary inoculum. The fungus is usually associated with the hilum area of the seed coat. Contamination may be external o internal and affects viability of seed with the time. Has been not found consistent correlation between disease severity on pods and incidence on seed infection and seed transmission of P. griseola is an insignificant o minor source of primary inoculum [Correa et al., 1989].
The most important source of primary inoculum for the ALS disease is pathogen-infected plant debris in the field. Pathogen viability decreases rapidly in plats debris buried beneath the soil surface [Correa et al., 1989].
Detection/indexing method at CIAT
- The fungus can be detected by incubating seeds on either agar or wet blotters at 24°C [Frison et al., 1990] although Blotter test method [Kameswara et al., 2006] is more useful for bean followed for a mycological characterization throughout stereoscopy and optical microscopy.
Treatment/control
- The control by cultural practices include, crop rotation of at least two years between beans crops, planting in well-draining soil, removal of infected crop debris by plowing or other means, and planting pathogen-free seed [Correa et al., 1989]. For seeds, storing for over one or two year kills the fungus completely [Frison et al., 1990; Neegaard, 1977].
- Chemistry control by foliar applications can be achieved with Ferban-sulfur-adherent combination, Zineb, Benomyl (0.13-0.25 g/l), and Thiophanate (2.0 g/l). Multiple sprays of the systemic fungicide Bitertanol increased yields by 33-41%. Chemical treatment of seed is a useful approach for contaminated seed lots. Treatment with Benomyl (6 g/kg seed) and a Captan-Zineb combination (3.7 g/kg seed) applied in water-based slurry (0.11 g/ml) and a dry-treated with Steeped in a 1% solution of mercuric chloride for 30 minutes effectively eradicated the fungi from contaminated seed [Correa et al., 1989; Frison et al., 1990].
- At CIAT biological control assays with crude extract of Clitoria tenatea (L.) seeds showed antifungal activity In vitro against the pathogen P. griseola. Conidia treated with crude protein extract of this seed failed to germinate after treatment, whereas those treated with sterile water germinated and converted into mycelia [Kelemu et al., 2005].
Procedure in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Allorent D, Savary S. 2005. Epidemiological Characteristics of Angular Leaf Spot of Bean: A Systems Analysis. European Journal of Plant Pathology. 113:329–341.
Biolib. 2009. http://www.biolib.cz/en/image/id67281/
Correa VFJ, Pastor-Corrales MA, Saettler AW. 1989. Angular leaf spot. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 59-75.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JDJD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Kelemu S, Mahuku GS, Segura G. 2005. An antifungal protein of the tropical forage legume Clitoria ternatea controls disease under field and greenhouse conditions [abstract]. Phytopathology (USA) 95(6):S52.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Melzer MS, Boland GJ. 2001. First Report of Angular Leaf Spot Caused by Phaeoisariopsis griseola on Bean in Ontario, Canada. Plant Disease (USA) 85 (8): 919-919.
Neergaard P. 1977. Seed pathology. John Wiley, New York, NY, USA.
Scientific name
Rhizoctonia solani Kühn (telemorph: Thanatephorus cucumeris (Frank) Donk)
Significance
Rhizoctonia solani is a destructive soil-borne pathogen attacking many crops worldwide and has economic impact in all soybean producing areas. Rhizoctonia root rot is a common root rot disease of beans in Latin America and the world. Losses of more than 10% have occurred in the United States and infection nearly 100% in bean planting in Colombia (near Popayan), the coastal areas of Peru [Abawi, 1989].
Symptoms
Rhizoctonia solani may induce seed rot, damping-off, stem canker, root rot, and pod rot. Rhizoctonia can infect seeds before germination, resulting in seed decay. Lesions on a young seedling expand rapidly and result in damping-off. Seed and seedling infection reduce seedling establishing and therefore lower plant densities often severely enough to be visually [Abawi, 1989].
The fungus can be seed transmitted in beans. R. solani infect pods in contact with the soil surface, causing water-soaking, the characteristics reddish brown sunken lesions, and distinct margins around the lesions. Minute brown sclerotia may develop on the surface of, or embedded in, these cankers. These lesions may serve as an inoculum source for infection beans in transit and ensure fungus dissemination as well as causing seed discoloration [Abawi, 1989].
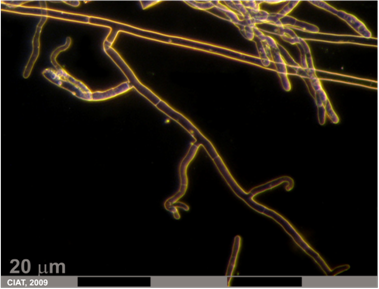 Rhizoctonia root rot (photo: CIAT) |
Hosts
The pathogen have a large number of host species and having reported in seeds of Brassica spp., Capsicum spp., Citrus spp., Gliocladium virens, Gosypium spp., Lycopersicon esculentum, Phaseolus spp., Spinacia oleracea, Vignia ungiculata, Zea mays, Zinnia elegans [Neergaard, 1977].
Geographic distribution
In Latin America, R. solani occurs in Mexico, all countries of Central America, and the Caribbean, and in South America in the Amazon region of Peru and Brazil, the coffee Zone of Colombia, and the northwestern region of Argentina. Other countries that have reported the disease are USA, Japan, Philippines Burma, and Sri Lanka, and as a minor pathogen in Kenya and Malawi [Gálvez et al., 1989].
Biology and transmission
In nature, R. solani exist as many strains, differing in cultural appearance, physiology, and pathogenicity. Naturally occurring strains or isolates differ in mycelium, grow rate, saprophytic behavior, and enzyme production [Abawi, 1989].
Teleomorph, Thanatephorus cucumeris, may occur and form a hymenial layer at the base of plants and/ or the underside of soil aggregates during periods of high humidity and rainfall. Basidia are short and barrel shaped with stout straight sterigmata while basidiospores are smooth, thin walled, and hyaline [Abawi, 1989].
Detection/indexing method at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy.
Treatment/Control
- Because R. solani has a worldwide distribution, including in uncultivated soils, exclution and eradication usually are not effective field control measures. The fungus can be eradicated from infected greenhouse soil by steaming at 60° C for 30 minutes. R. solani infection may be reduced by various cultural practices. In Colombia the infection is less severe during the wet rainy season if bean are planted on raised beds that facilitate good drainage. Seedling injury is minimized by shallow planting so that less seedling tissue is exposed to inoculum. Seeds planted 7.5 cm deep developed more root rot and hypocotyl injury than seed planted only 2.5 cm deep [Abawi, 1989].
- Continuous planting of beans in the same field increases the inoculum density of Rhizoctonia solani. However, crop rotation with non host crops reduces the incidence of bean root rot even though it does not completely eradicate the pathogen. Fungus population rapidly decline in soil planted with wheat, oats, barley or maize. Population levels remain relatively high in soil planted with susceptible bean, pea, or potato plants. An alternative to crop rotation would be the incorporation of selected residue or decomposable material. Also many antagonists or mycoparsites as Trichoderma species, have been effectively reduced activities of R solani when incorporated with organic amendments or directly on seed. Deep plowing is another cultural practice that is effective in reducing surface inoculum of R. solani and thus disease incidence. Turning under soil and crop residue to a deep 20-25 cm has reduced Rhizoctonia root rot on beans for three years [Abawi, 1989].
- Fungicides that are effective against R. solani include PCNB (the most commonly used fungicide to control R. solani), Benomyl, Carboxin, Busan 30A, Thritam, Zineb, Chloroneb, and others. These fungicides are commonly applied as seed treatments (1-3 g i.a./ kg seed) before or during planting [Abawi, 1989].
- Biological control with Trichoderma harzianum, Bacillus subtilis and B. licheniformis reduced Rhizoctonia root rot. Seed treatment and root drenching with bacterial suspensions with 0.5% chitin was more effective against Rhizoctonia solani in Capsicum annuum [Sid Ahmed et al., 2003] than addition of the organisms without chitin.
Procedure in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Abawi GS. 1989. Root rots. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 105-157.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Neergaard P. 1977. Seed pathology. John Wiley, New York, NY, USA.
Sid AA, Ezziyyani M, Sánchez CP, Candela ME. 2003. Effect of chitin on biological control activity of Bacillus spp. and Trichoderma harzianum against root rot disease in pepper (Capsicum annuum) plants. European Journal of Plant Pathology 109 (6):633.
Scientific names
Fusarium solani (Martius) Appel and Wr. f. sp. phaseoli (Bruk.) Snyd. & Hans.
Significance
Fusarium root rot of beans is caused by Fusarium solani (Martius) Appel and Wr. f. sp. phaseoli (Bruk.) Snyd. & Hans. The pathogen is prevalent and causes varying degrees of damages in most bean growing areas of the world. The disease has reported yield loss until of 86%, but more commonly in ranged from 6-53%, depending upon the bean cultivar and other stress factors [Abawi, 1989].
Symptoms
Initial symptoms of fusarium root rot appear as longitudinal, narrow, reddish lesions or streaks on the hypocotyl and primary root about one or two weeks after seedling emergence. As infection progresses, lesions become numerous, coalesce, and the entire underground stem and root system may become covered with reddish brown superficial lesions. The discoloration may extend to the soil surface, but rarely beyond. The lesions have not definite margins and may be accompanied by longitudinal fissures [Abawi, 1989].
The primary and lateral the primary and lateral roots are frequently killed by the fungus and may remain attached as decomposed and dried remnants. When the primary root is killed, the lower stem may become pithy or hollow. There is not pronounced wilting symptoms although severely infected plants are stunted, chlorotic, and exhibit premature defoliation. Lateral adventitious roots often develop above the initial lesion areas and support plant growth so that a crop yield is still produced although later become similarly infected and sometimes are killed by the pathogen. Also, pod number per plant and seed size may be reduced [Abawi, 1989].
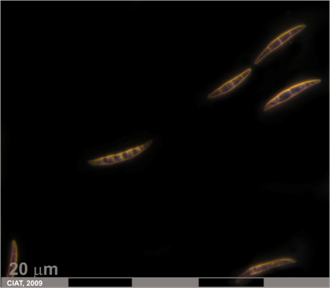 Fusarium root rot (photo: CIAT) |
Hosts
Fusarium solani attacks diverse species of beans as Phaseolus vulgalis L.; P. lunatus L.; P. coccineus L.; Vignia angularis (Willd.) Ohwi et Ohasi; V. aconitifolia (Jacq.) Maréchal; V. unigulata (L.) Walpers subsp. ungiculata; Pisum sativum L.; Onobrychis viciifolia Scop.; and Pueraria lobata (Willd.) Ohowi [Abawi, 1989].
Geographic distribution
Cosmopolitan
Biology and transmission
Chlamydospores of F. solani f. sp. phaseoli, either associated with infected bean tissue or free in soil, are often under the influence of soil fungistasis. They can therefore remain dormant in soil with little mobility for a long time. When soil fungistasis is reversed, chlamydospores germinate where bean seed or rot exudates are available. The pathogen was reported to directly penetrate bean tissue or enter through stromata and wounds. After penetration, the fungus grows intercellulary throughout cortical tissues, but is stopped, by the epidermis layer [Abawi, 1989]. Some distinguishing characteristic are the morphology of the macroconidia, the elongate monophialides bearing microconidia, which also help distinguish it from F. oxisporum, and the distinctive cream, blue-green or blue color of conidies on PDA [Nelson et al, 1983].
The pathogen is disseminated within and between bean fields by such means as movement of infected soil, infected host tissues, colonized debris, drainage and irrigation water, contaminated bean seed. Once introduced into a field, this pathogen becomes uniformly at high densities after two or three bean crops. The pathogen is also capable of colonizing organic matter under certain environmental conditions, therefore maintaining or increasing its population in absence of bean [Abawi, 1989].
Stress factors that aggravate fusarium root rot and its damage to beans include soil compaction, excess soil moisture, drought, high density plantings, herbicide damage, the ammonium of nitrogen fertilizers, toxic metabolites of decomposing crop residue, and soil temperatures unfavorable for bean seed germination and growth [Abawi, 1989].
On PDA grown is rapid, often with abundant aerial mycelium. The surface is soon covered with confluent sporodochia that give the appearance of pionotes and color the surface cream, blue-green, or blue, but never orange. Some clones may show a dark purple color on the upper surface. The under surface is generally colorless, but some clones produce a dark violet pigment [Nelson et al, 1983].
Detection/indexing method at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy with specialized literature [Nelson et al, 1983].
Treatment/control
- When virgin soils are to be used for bean production, all measures to be employed to prevent to introduction of the pathogen into these soils. Eradication on large scale is uneconomical and impossible once the pathogen becomes established within the field. Well-drained and well-fertilized soils promote vigorous plant growth. Shallow cultivation prunes laterals roots, which usually from above infected hypocotyl tissues, and most be avoided in heavily infected plantings. Hilling up soil around the stem of infected plants promote adventitious root formation reducing root-rot damage [Abawi, 1989].
- Long term crop rotation with non susceptible plant such as wheat and barley lowers soil populations of F. solani f. sp phaseoli and reduce damage to beans. Soil amendment with various crop residues with high carbon to nitrogen ratios such as small grains and maize, may reduce root-rot damage [Abawi, 1989].
- Cultural practices that reduce soil compaction and loosen hard pans are most effective in reducing root-rot damage to beans. Secondary tillage that encourages soil compaction decreases colonization of beans by symbiotic vesicular arbuscular mycorrhizal fungi.
- Control by chemicals as Thiram, PCNB, Benomyl (0.56 kg/ha), Captafol (4.7l/ha), and Busan 30A, reduce fusarium root-rot severity on hypocotyls and roots of young seedlings [Abawi, 1989].
- The herbicide Trifluralin, Bentazol, and Avadex and the insecticides Metasystox and Nicotine stimuli growth of F. solani f. sp. phaseoli and may increase root-rot damage [Abawi, 1989].
Procedure in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Abawi GS. 1989. Root rots. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 105-157.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Nelson PE, Toussoun TA, Marasas WFO. 1983. Fusarium species: An illustrated manual for identification. Pennsylvania State University Press, University Park, PA, USA. 193 p.
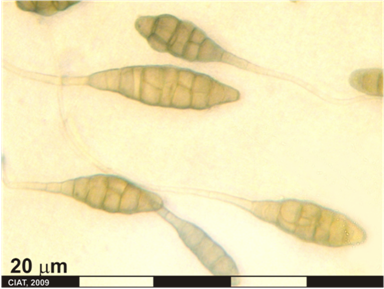
Scientific name
Fusarium oxysporum Schlecht. f. sp. phaseoli Kendrick and Snyder
Significance
Fusarium yellow of bean is caused by Fusarium oxysporum Schlecht. f. sp. phaseoli Kengrick and Snyder [Abawi, 1989]. F. oxysporum is the most important plant pathogenic species of Fusarium, having a wide host range, and including numerous formae specials, some of which contain two or several pathogenic races causing devastating wilt diseases, and many of which are seed-borne [Neergaard, 1977].
Symptoms
The Fusarium yellows pathogen is morphologically similar to all the members of the species F. oxysporum. However, it is recognized by its physiological and pathological adaptation to beans, hence the interspecific taxa designation f. sp. (formae speciales) phaseoli. Initial symptoms appear on lower leaves which exhibit yellowing and wilting. These symptoms may be confused with those caused by phosphorous deficiency. This yellowing and wilting becomes more pronounced and progress upward into younger leaves. Stunting may also become evident, especially if plant infection occurred during the seedling state [Abawi, 1989].
The margin of infected leaves may become necrotic and diseased plants become progressively more chlorotic. The fungus also can cause water-soaked lesions on pods. Severely plants infected exhibit permanent wilting and premature defoliation. The characteristic pink-orange spore masses of fungus may appear on stem and petiole tissue. Vascular discoloration is the diagnostic symptom and is usually evident after the initial appearance of foliar symptoms [Abawi, 1989].
Hosts
The main host for Fusarium oxysporum f. sp. phaseoli is the Phaseolus spp., however, the species have a great number of host as Allium, Asparragus officinalis, Beta vulgaris, Bromus, Callisthepus chinensis, Cannabis sativa, Citrulus vulgaris, Cucumis sativus, Glyricine max, Gossypium, Lens culinaris, Linum grandiflorum, L. usitatissimun, Lupinus luteus, Lycopersicom esculentum, Matthiola incana, Medicago sativa, Oryza sativa, Phaseolus vulgaris, Pisum sativum, Psuedotsuga menziesii, Solanum melongena, Sorghum vulgare, Spunacia oleracia, Tegetes, Trifolium pretense, T. repens, Vicia fava, Vignia ans Zea mays [Neergaard, 1977]
Geographic distribution
Cosmopolitan
Biology and transmission
This pathogen has been associated with seed a surface contaminant, and is capable of penetrating intact root tissue, usually near the root tip and just behind the root cap. After penetration, hyphae of the pathogen move inter-and intracellularly and invade the developing xylem vessels. Penetration of older parts of roots and hypocotyl tissue occurs, usually trough wounds or natural openings. The fungus is confined to xylem vessels until the later stages of disease development, although limited invasion of xylem parenchyma tissue may occur. Infection appears to proceed between xylem vessels in susceptible cultivars, through hyphal growth, and through the transport of newly formed by the transpirational stream. The latter include vascular occlusion by the formation of gel plugs, tyloses, deposition of additional wall layers, and infusion of these structures with phenols and other metabolites. At later stage of disease development, pathogens grow into adjacent cortical tissue, producing large number of chlamydospores. The fungus may also emerge on the surface on infected plant tissue, producing abundant pink mycelia growth and conidia [Abawi, 1989].
On PDA growth is rapid and the white aerial mycelium may become tinged with purple or be submerged by the blue color of the sclerotia when they are abundant, especially at the base of the slant, or by the cream to tan to orange sporodochia when these are abundant. Discrete erumpent orange sporodochia are present in some strain. The undersurface may be colorless to dark purple, and these colors may be visible through the mycelium when viewed from above [Nelson et al, 1983].
Detection/indexing method in place at the CGIAR Center at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy and taxonomic claves throughout specialized literature [Nelson et al, 1983].
Treatment/control
- Cultural and chemical control measures reported for F. solani f. sp. phaseloi, especially crop rotation and fungicide seed treatments, are also applicable for fusarium yellows on beans. The most effective control measure against fusarium yellows is the use of resistant cultivars [Abawi, 1989].
Procedure in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Abawi GS. 1989. Root rots. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 105-157.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Neergaard P. 1977. Seed pathology. John Wiley, New York, NY, USA.
Nelson PE, Toussoun TA, Marasas WFO. 1983. Fusarium species: An illustrated manual for identification. Pennsylvania State University Press, University Park, PA, USA. 193 p.
Scientific names
Sclerotium rolfsii Sacc. (Teleomorph, Athelia rolfsii (Curzi) Tu & Kimbrough)
Significance
This disease is caused by Sclerotium rolfsii Sacc. (Teleomorph Athelia rolfsii (Curzi) Tu and Kimbr.). Direct estimates of yield losses caused by this pathogen in beans are not available [Abawi, 1989].
Symptoms
The infectious process can result in damping-off, stem blight, and root rot. Initial symptoms on infected plants appear as dark-brown water-soaked lesions on the lower stem surface area just below the soil line. These lesions extend downward, through stem tissue and so start root-rot symptoms. Under moist conditions, lesions on the stem tissue continue to progress downward and eventually may kill the entire root system [Abawi, 1989].
Other symptoms consist of leaf yellowing and defoliation of the upper plant branches which may be followed by a sudden wilt condition. Abundant white coarse mycelium and sclerotia and soil particles are often found attached to stem tissue near the line soil [Abawi, 1989].
Hosts
Sclerotium rolfsii has a wide host range of more than 200 species of plants, involving most vegetable crops and including beans [Abawi, 1989].
Geographic distribution
The disease occurs in many warm and humid areas located between the northern and southern 38° latitudes [Abawi, 1989].
Biology and transmission
The fungus grows readily on host residue on the soil surface under favorable environmental conditions. It produces white and coarse mycelium and numerous characteristic sclerotia that are smooth walled, round (0.5-1.5 mm in diameter), and brown. Sclerotium rolfsii does not produce asexual spores and the basidial state, is rarely produced in culture or in field [Abawi, 1989].
Sclerotia of S. rolfssi survive in soil for at least one year. The fungus can also survive in infected host tissue and saprophytically by colonizing available organic residue. High moisture and temperature are required for optimal growth and reproduction of the fungus in the soil. This pathogen is sensitive to low temperature and rarely occurs in bean-growing areas with cold periods. Sclerotial germination is induced by volatiles which emanate from crop residue in the soil and is enhanced by wet and dry conditions [Abawi, 1989].
Dispersal of the pathogen may occur through contaminated irrigation water, infested soil, adhering to agricultural tools and animals, or contaminated seed. Sclerotia can be transported relatively long distances by animals due to they can pass through the digestive tract of animals without losing viability [Abawi, 1989].
Detection/indexing method at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy.
Treatment/control
- Control measures that exclude introduction of S. rolfsii into clean fields or infected plants material, should be practiced, furthermore eradication of susceptible weed hosts and destruction of infected host residue by burning or deep plowing will reduce soil population densities of S. rolfsii and, therefore, disease potential.,/li>
- Biological controls assays have been made with Penicillium sp. and Trichoderma harziarum under greenhouse and field conditions respectively [Abawi, 1989].
- The use of chemicals for control the fungus including PCNB, Captafol, Fentin, and Tridemorph, has been applied as soil treatments. Although, sclerotia are difficult to destroy with fungicides. The herbicide Eptam, aggravated the damage caused by S. rolfsii to ladino clover and cotton and reduced the biocontrol activity of Trychoderma viridae [Abawi, 1989].
Procedure in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Abawi GS. 1989. Root rots. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 105-157.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
 |
 |
|
|
Southern blight (photos: CIAT) |
||
Scientific name
Rhizoctonia solani Kühn
Significance
The disease is caused by the fungus Rhizoctonia solani Kühn, the state sclerotial, or asexual, stage of the basidiomycete fungus Thanatephorus cucumeris (Frank) Donk. The fungus is mainly soilborne that is widely distributed throughout the world [Gálvez et al., 1989]. ., 1989]. infects bean plants by colonizing senescent and dead organs such as blossoms, cotyledons, seeds, leaves, or injured plant tissue. The disease show cotyledonary rot on bean seedlings which developed from mycelia-or-sclerotia-infested seed lots planted in the greenhouse. After colonizing a senescent plant organ, the fungus enters the hosts by mechanically disrupting the cuticle. It used a dome-shaped infection cushion which had developed from an appressorium. Large vesicles from between the cuticle and epidermal layers and infection hyphae develop intercellulary. Hyphae brand from the infection hyphae and ramify inter-and intracellularly, causing a watery soft rot.
Web blight is a very important bean-production problem in the humid lowland tropics in Latin America and the Caribbean, where warm to high temperatures and abundant rainfalls prevail [Gálvez et al., 1989].
Under field conditions web blight can occur at any stage of the bean-crop cycle and cause severe blight, resulting in rapid defoliation and often complete crop failure [Gálvez >em>et al
Symptoms
It causes a diversity of diseases such as seed decay, root-and-hypocotyl rot, and foliar blight. Even though morphologically similar, some isolates causes aerial infection such as web blight of beans, while others attack only roots and hypocotyls.
Web blight symptoms initiated by rain-splashed sclerotia or mycelium fragments differ from those elicited by basidiospores. Sclerotia germinate during periods of favorable environmental conditions by producing hyphae, a few mm in length, that branch profusely until they reach host tissue. Sub-epidermal hyphae develop inter-and intracellularly. Lesions first appear on the primary leaves as small necrotic spots with brown centers and olive-green spots. Under favorable environmental conditions they progress very rapidly but appear irregular and somewhat zonate. Often these lesions coalesce and affect the entire leaf. Infected leaves rapidly become covered by small sclerotia and mycelium [Gálvez et al., 1989].
The light-brown superficial hyphae spread in a fan-shaped manner on either leaf surface. Hyphae may grow rapidly over healthy leaves, petioles, flowers, and pods. Small brown sclerotia form three to six days after infection. Many lesions produced by basidiospores are distinct small, necrotic, circular, and measure 2-3 mm in diameter. They are light brown and brick red with a lighter center. Under humidity conditions, these spots fall from the leaf surface, resulting in a symptom known as “cock’ eye”. Pod lesions caused by sclerotia, mycelium, or basidiospores are also small, circular and have light-brown centers surrounded by a reddish brown darker border [Gálvez et al., 1989].
Bean pods may become infected during the grain-filling stage. Young pod infections appear as light-brown, irregular-shaped lesions which frequently coalescence and kill the pod. Seeds can become infected in the endosperm and radicular end of the embryo and on the seed-coat surface [Gálvez et al., 1989].
Hosts
R. solani is a pathogen of a large number of host species including bean, beet, cabbage, carrot, cucumber, eggplant, melon, soybean, tobacco, tomato, watermelon, and many crop plants [Gálvez et al., 1989] as Brassica spp., Capsicum spp., Citrus spp., Gosypium spp., Lycopersicon esculentum, Phaseolus spp., Spinacia oleracea, Vignia ungiculata, Zea mays, Zinnia elegans [Neergaard, 1977].
Geographic distribution
Rhizoctonia solani has a worldwide distribution. In Latin America, web blight occurs in Mexico, all countries of Central America, and the Caribbean, and in South America in the Amazon region of Peru and Brazil, the coffee Zone of Colombia, and the northwestern region of Argentina. Other countries that have reported the disease are USA, Japan, Philippines Burma, and Sri Lanka, and as a minor pathogen in Kenya and Malawi [Gálvez et al., 1989].
Biology and transmission
Both stages (sclerotial and basidial) can initiate the disease, although they cause different symptoms. Web blight epidemics are favored by rainy weather, high (30° C) to moderate (20° C) air temperature, high to moderate soil temperature, and high relative humidity of the least 80%. The main sources of inoculum that can initiate infection are sclerotia and mycelium fragments, either free in the soil or present on colonized debris. Plants are inoculated with the pathogen when raindrops splash soil particles, infested with sclerotia or mycelium, onto plants. Infection caused by basidiospores is less abundant and do not contribute significantly to epidemic development, particularly when lesions from basidiospore infection appear late in the crop cycle [Gálvez et al., 1989].
Infected bean seed can disseminate the pathogen over long distances, introduce it into new fields, or act as a source of primary inoculum. When rain splashed sclerotia and mycelium is the main source of inoculum, initial symptoms of web blight always appear on primary leaves two weeks after planting [Gálvez et al., 1989].
Basidiospores are dispersed during the night and remain viable for only a few hours. Sclerotia can remain viable in soil for several years and can survive as vegetative mycelium within plant residue [Gálvez et al., 1989].
Detection/indexing method
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy.
Treatment/control
- Control by cultural practices includes planting seed free of internal or external contamination, sanitation of infected crop debris, and crop rotation with nonhost such as tobacco, maize, and grasses. The mulch is a practice most effective, that forms a barrier and impedes the splashing of pathogen propagules from the soil infected to plant tissues. However, mulches may create more favorable conditions for slug infestation and resulting crop loss in some production regions [Gálvez et al., 1989].
- Benomyl (0.25-0.5 kg/ha) helps manage the pathogen when it is applied at first-symptoms appearance and then every 15 days. Fentin acetate (0.16 kg/ha) or Fentin hydroxide (0.20 kg/ha) applied after benomyl, gives good control. Thiophanatemethyl (0.5 kg/ha), Carbendazim (0.5-1.0 kg/ha), and Captafol (1.0-3.5 kg/ha) are also useful [Gálvez et al., 1989].
Procedure followed in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Gálvez EGE, Mora B, Pastor-Corrales MA. 1989. Web blight. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 195-209.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p. Neergaard, Paul. 1977. Seed pathology. John Wiley, New York, NY, USA.
Scientific name
Sclerotinia sclerotiorum (Lib.) de Bary
Significance
The white mold fungus, Sclerotinia sclerotiorum (Lib.) de Bary, is most important in templerate zones of the northern and southern hemispheres. However is also a problem in areas with tropical or arid climates, especially during cool seasons or under favorable microclimatic conditions.
Symptoms
S. sclerotiorum infects bean plants by colonizing senescent and dead organs such as blossoms, cotyledons, seeds, leaves, or injured plant tissue. The disease show cotyledonary rot on bean seedlings which developed from mycelia-or-sclerotia-infested seed lots planted in the greenhouse. After colonizing a senescent plant organ, the fungus enters the hosts by mechanically disrupting the cuticle. It used a dome-shaped infection cushion which had developed from an appressorium. Large vesicles from between the cuticle and epidermal layers and infection hyphae develop intercellulary. Hyphae brand from the infection hyphae and ramify inter-and intracellularly, causing a watery soft rot.
Symptoms of infection first appear as a water-soaked lesion, followed by a white moldy growth on the affected organ. This infected tissue later become dry, light colored, and assumes a chalky or bleached appearance. Plant wilting may also be seen within the plant canopy after plant stems and/ or vines are infected.
 White mold (photo: Igzev, 2009.) |
Hosts
Sclerotinia sclerotiorum is pathogenic o a wide range of host plants included 374 species of 237 genera. Hosts are as diverse as ornamentals, tree fruits, vegetables, oil-seed crops, and legumes.
Geographic distribution
The fungus is a cosmopolitan fungus and occurs in regions where conditions are favorable such as moisture and low temperature. S. sclerotiorum has been reported in Argentina, Brazil, Mexico, Colombia, Venezuela, Peru, others areas of Latin America, Asia, Africa, Europe, Australia, and North America.
Biology and transmission
Fields used repeatedly for bean production, even in short crop rotations, will often contain many sclerotia. Sclerotia formed on or within diseased tissue may be dislodged onto the soil surface by wind or harvesting operations. Subsequent land preparation redistributes them within the soil profile and over the field.
Sclerotia also can be distributed by furrow irrigation within fields and by reuse of irrigation runoff water between fields. They can survive in sandy loam soils for at least three years and are capable of producing secondary sclerotia.
Detection/indexing method at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy.
Treatment/control
- Many soil organisms associated with S. sclerotiorum may degrade or decrease germinatation of sclerotia, such a as the fungi Coniothyrium minitans, Trichoderma sp., Aspergillus sp., Penicillium sp., Fusarium sp., Mucor sp., Sporidesmiun sclerotivorium, Teratosperma oligocladium, Gibberella baccata, Streptomyces sp., and bacteria. However, none of these agents has been used effectively in controlling S. sclerotiorum incidence or in protecting bean plants from infection under field conditions.
- For controlling the pathogen, cultural practices such as crop rotation, flooding, reduced seedling rates, fewer irrigations, and destruction of those bean-cull screenings which contain sclerotia are recommended.
- Chemical control with Benomyl, DCNA or Dicloran Dichlone, PCNB, or Thiabendazole around early-to mid –bloom controls S. sclerotiorum infection on snap and common beans, particularly under dryland conditions. Other fungicides such as Vinclosolin, Procymidone, and Iprodione, were tested for their effectiveness in controlling white mold.
Procedure followed at the centers in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Igzev. 2009. http://www.igzev.de/englisch/projekte.php?abteilung=4
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Schwartz HF, Steadman JR. 1989. White mold. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 211-230.
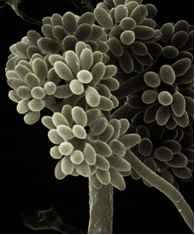
Scientific name
Alternaria spp.
Significance
The disease is caused by various fungi of the Alternaria species, including Alternaria alternata (Fr.) Kreisser), A. brassicae f. phaseoli Brun.; A. fasiculata (Cke. et Ell.) L. R. Jones et Grout; A. tenuissima (Nees ex Fries) Wiltshire; A. macrospora Zimm.; and A. brassicola (Schw.) Wiltsh. Severe epidemics may cause premature defoliation but yield losses are not usually significant [Schwartz, 1989].
Symptoms
Leaf symptoms appear as small, gray to reddish brown, irregular-shaped spots or flecks which may be water-soaked and surrounded by a darker brown border. These lesions gradually enlarge and develop as concentric rings that become brittle and fall out, producing a shot-hole appearance. Lesions may coalescence and cover large areas of the leaf, resulting in partial or premature defoliation [Schwartz, 1989].
Alternaria spp. can cause death of the central growing point on the plant or reduce plant vigor. The fungus also can blemish leaves and pods by producing a brown discoloration on the surface; it also damages developing seeds. The reddish to dark brown or black flecks may coalescence and produce streaks on infected pods. The pathogen is seed-borne and disease can be high if infection occurs near maturity [Schwartz, 1989]. Common bean seeds become infected whit Alternaria from distinct species promoted seed infection varying from 0 to 75% and the majority of these isolates was transmitted to seedlings [Moraes, 2006].
 Alternaria leaf-and-pod spot (photo: CIAT) |
Hosts
Phaseolus spp. [Schwartz, 1989].
Geographic distribution
These fungi are reported from East Africa, Brazil, Costa Rica, Colombia, Chile, Mexico, Venezuela, England, Canada and United States [Schwartz, 1989].
Biology and transmission
Alternaria spp. are wound parasites. They usually form lesions only on older or senescent plant tissue during periods of high humidity that last for three or four days and are relatively cool. However A. tenuis can also penetrate the leaf directly or through stomata. The fungus produces a toxin (tentoxin) in culture which induces plant chlorosis when applied to roots. However it does not produce detectable quantities of toxin during natural infection of laves or pods [Schwartz, 1989].
Detection/indexing method at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization through stereoscopy and compound microscopy. However, the ISTA have a method for detection of Alternaria padwickii on Oryza sativa and its similar to used in GHL [ITSA, 2008].
Treatment/control
- Control measures are seldom necessary buy consist of wider plant and row spacing, use chemicals, development of resistant cultivars, and crop rotation. Chemical control uses Chlorothalonil (1200 µg a.i./l), Thiophanate (2 g/l), and Zineb (2.4 g/l) reducing disease severity and increases yield in the susceptible cultivar [Schwartz, 1989].
Procedure followed at the centers in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
de Moraes MHD. 2006. Transmission of Alternaria spp. by common bean seeds and its effects on physiological quality. Summa Phytopathologica 32 (4):381-383.
International Seed Testing Association (ISTA). 2008. Annexe to Chapter 7: Seed Health Testingg Methods. 7-012: Detection of Alternaria padwickii on Oryza sativa (Rice).
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Schwartz HF. 1989. Additional fungal pathogens. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 231-259.
Botrytis Head Blight; Gray mold of beans, Stem Rot by Botrytis cinerea
Scientific name
Botrytis cinerea Pers. (Teleomorph. Sclerotinia fuckeliana (de Bary) Fuckel)
Significance
Botrytis cinerea Pers. is an important pathogen of many crops.
Symptoms
Lesions develop as brown, water-soaked areas becoming greyish on drying in the inflorescence with extensive blossom blighting and apical dieback. Under humid conditions, profuse sporulation occurs on inflorescences and stems. Buff-coloured stem lesions develop where diseases flower parts lodge.
 Botrytis cinérea (photo: Universitat d'Alacant) |
Hosts
Stylosanthes spp., it has been found on S. guianensis, S. hamata, S. humilis and S. viscose, beans and many other crops.
Geographic distribution
Cosmopolitan
Biology and transmission
Conidia are mainly airborne and may also be carried by raindrop splash. Diseases inflorescences, on which sporulation is profuse under wet conditions, are important sources of inoculum in epidemics. The fungus survives as sclerotia or as mycelium associated with host lesions.
Detection / indexing method in place at the CGIAR Center at CIAT
- PDA Test and direct visualization in Stereomicroscopy and Microscopy. The ITSA has a method used that is similar to used for GHL [ITSA, 2008].
Treatment/control
- In bean the disease has been controlled by foliar applications of the fungicide benomyl during bloom, however some strains of B. cynerea develop resistance.
- Biological control with saprophytic yeast (one isolate of Rhodotorula glutinis and two isolates of Cryptococcus albidus) effectively controlled diseased in bean and tomato plants.
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration seed process in field.
References and further reading
Elad Y, Köhl J, Fokkema NJ. 1994. Control of infection and sporulation of Botrytis cinerea on bean and tomato by saprophytic yeasts. Phytopathology (USA) 84(10):1193-1200
International Seed Testing Association (ISTA). 2008. International Rules for Seed Testingg Annexe to Chapter 7: Seed Health Testingg Methods. 7-003: Detection of Botrytis cinerea on Helianthus annuus (Sunflower).
La Mondia JA, Douglas SM. 1997. Sensitivity of Botrytis cinerea from Connecticut Greenhouses to Benzimidazole and Dicarboximide Fungicides. Plant Disease 81(7): 729.
Lenné JM. 1994. Diseases of Aeschynomene. In: Lenné JM, Trutmann P, editors. Diseases of tropical pasture plants. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI: Oxon, GB. Pp. 97-107.
Lenné JM. 1994. Diseases of Stylosanthes. In: Lenné JM, Trutmann P, editors. Diseases of tropical pasture plants. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB. Pp. 21-42.
Universitat d'Alacant. 1996-2009. Unidad de Diagnóstico y Control Biológico de Enfermedades Vegetales. http://www.ua.es/secretaria.gral/es/memoria/1998_99/ix_12_42.htm (Accessed on July 2009).
Zalewski JC, Johnson ER. 1977. Benlate tolerance in gray mold beans. Proc. Oerg. Hortic. Soc. 68: 95-97. Cited in: Johnson, K.B., Powelson, M.L. 1983. Analysis of spore dispersal gradients of Botrytis cinerea and gray mold disease gradients in snap beans. Phytopathology 73(5):741-746.
Ascochyta Leaf Spot, Ascochyta Blight
Scientific names
Phoma exigua var. diversispora (Bubák) Boerema and Phoma exigua var. exigua (Sacc.)
Significance
Disease is caused by Phoma exigua var. diversispora (Bubák) Boerema and Phoma exigua var. exigua Sacc. Ascochyta blight of beans, also known as Ascochyta leaf-and-pod spot, is a fungal disease of economic importance only in regions with cool humid conditions such as those found at elevations above 100 m in the Andean region of South America [Schwartz, 1989].
Symptoms
Infection P. exigua var. diversispora is favored by high humidity, continuous rains accompanied by winds, and cool to moderate temperatures. Symptoms first appear on leaves. They are black, concentric, zonate lesions, 1-3 cm diameter, and may later contain small black pycnidia. These dark to black lesions also may appear on the peduncle, petiole, node and pod, and can cause stem girdle and plant death. The fungus may also spread systemically throughout the plant. Premature leaf drop may occur during severe epidemics and the fungus is seed borne [Schwartz, 1989].
Symptoms with P. exigua var. exigua are very similar and include brown-to-black lesions on infected leaves, which developed concentric zones 10 to 30 mm in diameter and also contained small, black pycnidia. Concentric dark gray-to-black lesions also appeared on branches, stems, nodes, and pods. Infected seeds turned brown to black. Plants sometimes showed defoliation and pod drop [Bardas et al., 2008].
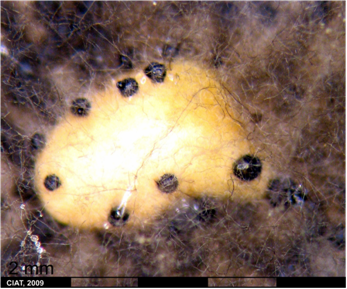 Ascochyta Leaf Spot (photo: CIAT) |
Hosts
Found throughout the world, causing diseases in a large number of crop species [Frison et al, 1990].
Geographic dDistribution
Worldwide
Biology and transmission
The pathogens of this disease are called Phoma exigua var. diversispora (Bub.) Boerema. and Phoma exigua var. exigua Desmazieres, formerly known as Ascochyta phaseolorum Saccardo. It has been also reported as a less important pathogen associated with Ascochyta blight. Phoma exigua isolates produce hyaline, septate, submerged mycelium in culture [Schwartz, 1989].
Detection/indexing method in place at the CGIAR Center at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy.
Treatment/control
- Control measures are crop rotation, wide plant spacing, planting clean seed, chemical treatment of seed, and foliar application of sulfur fungicides.
- The fungicides include Benomyl (0.55 g/l), Zineb (2.4 g/l), Chlorothalonil (2.24 kg/ha), and Carbendazim [Schwartz, 1989; Frison et al, 1990; Trutmann, 1992].
Procedure followed in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Bardas GA, Tziros GT, Tzavella-Klonari K. 2008. First Report of Ascochyta Leaf Spot Caused by Phoma exigua var. exigua on Common Bean in Greece. Plant Disease 92 (4):653.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD, editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Schwartz HF. 1989. Additional fungal pathogens. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 231-259.
Trutmann P. 1992. Seed treatments increase yield of farmer varietal field bean mixtures in the central African highlands through multiple disease and beanfly control. Crop Protection 11 (5):458-464.
Ashy stem blight, Charcoal rot, Crown rot
Scientific name
Macrophomina phaseolina (Tassi) Goid
Other scientific names
Macrophomina phaseoli (Maub.) Ashby., Sclerotium bataticola Taub.
Significance
Ashy stem blight of bean is caused by the fungus Macrophomina phaseolina (Tassi) Goid. 1990]. The disease is more prevalent and damaging to beans that are exposed to drought and warm temperatures [Schwartz, 1989].
Symptoms
Symptoms may appear after soil-borne mycelia or sclerotia germinate and infect seedling stems near the soil line at the base of developing cotyledons. The fungus produced black, sunken, cankers which have a sharp margin and often contain concentric rings. The plant’s growing tip may be killed or the stem broken where it is weakened by the canker. Infection may continue into the hypocotyl and root region or the primary leaf petioles. Older seedling and plant infections may cause stunting, leaf chlorosis, premature defoliation, and plant death [Schwartz, 1989].
Hosts
This fungus attacks Phaseolus vulgaris L., P. lunatus L., soybean, maize, Sorghum, and many other crops [Schwartz, 1989] as Brassica napus L. [Khangura & Aberra, 2009], strawberry [Koike, 2008], Beta vulgaris L. [Karadimos, et al., 2002], Helianthus annuus L. [Gulya et al., 2002], Gossypium hirsutum L. [Baird & Brock, 1999] and Casssava [Msikita et al., 1998], white clover and alfalfa [Pratt et al., 1998].
Geographic distribution
Worldwide
Biology and transmission
The fungus produce black globose pycnidia that contain large, colorless, one-celled, fusiform conidia which are pointed at one end and rounded at the other end. Sclerotia and pycnidia also are produced on infected plants [Schwartz, 1989].
Detection/indexing method in place at the CGIAR Center at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy.
Treatment/control
- Control measures are planting clean seed, treating seed with chemicals such as Benomyl (1 kg/ha), and sanitation or deep-plowing plant debris containing pycnidia and sclerotia.organic soil amendments and high soil temperatures and moisture may reduce sclerotia levels [Schwartz, 1989].
- Seed treatment with fungicides such as Quintozene (PCNB) and Captan completely eradicates seed-borne infection without any adverse effect on seed germination [Frison et al., 1990].
Procedure followed in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Baird RE, Brock JH. 1999. First Report of Macrophomina phaseolina on Cotton (Gossypium hirsutum) in Georgia. Plant Disease 83 (5):487.
Bradley CA, del Río LE. 2003. First Report of Charcoal Rot on Soybean Caused by Macrophomina phaseolina in North Dakota. Plant Disease 87 (5):601.
ElAraby ME, Kurle JE, Stetina SR. 2003. First Report of Charcoal Rot (Macrophomina phaseolina) on Soybean in Minnesota. Plant Disease 87 (2):202.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD editors. 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Gaetán SA, Fernandez L, Madia M. 2006. Occurrence of Charcoal Rot Caused by Macrophomina phaseolina on Canola in Argentina. Plant Disease 90 (4):524.
Gulya TJ, Krupinsky J, Draper M, Charlet LD. 2002. First Report of Charcoal Rot (Macrophomina phaseolina) on Sunflower in North and South Dakota. Plant Disease 86 (8):923.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Karadimos DA, Karaoglanidis GS, Klonari K. 2002. First Report of Charcoal Rot of Sugar Beet Caused by Macrophomina phaseolina in Greece. Plant Disease 86 (9):1051.
Khangura R, Aberra M. 2009. First Report of Charcoal Rot on Canola Caused by Macrophomina phaseolina in Western Australia. Plant Disease 93 (6):666.
Koike ST. 2008. Crown Rot of Strawberry Caused by Macrophomina phaseolina in California. Plant Disease 92 (8):1253.
Mertely J, Seijo T, Peres N. 2005. First Report of Macrophomina phaseolina Causing a Crown Rot of Strawberry in Florida. Plant Disease 89 (4):434.
Msikita W, James B, Wilkinson HT, Juba JH. 1998. First Report of Macrophomina phaseolina Causing Pre-harvest Cassava Root Rot in Benin and Nigeria. Plant Disease 82 (12):1402.
Pratt RG, McLaughlin MR, Pederson GA, Rowe DE. 1998. Pathogenicity of Macrophomina phaseolina to mature plant tissues of alfalfa and white clover. Plant Disease. 82:1033-1038.
Schwartz HF. 1989. Additional fungal pathogens. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 231-259.
Yang XB, Navi SS. 2005. First Report of Charcoal Rot Epidemics Caused by Macrophomina phaseolina in Soybean in Iowa. Plant Disease 89 (5):526.
Zveibil A, Freeman S. 2005. First Report of Crown and Root Rot in Strawberry Caused by Macrophomina phaseolina in Israel. Plant Disease 89 (9):1014.
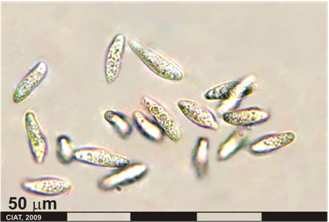 |
 |
|
|
Ashy stem blight (photos: CIAT) |
||
Cercospora leaf blotch, Cercospora leaf spot, Leaf spot of beans
Scientific names
Cercospora canescens Ellis Martin and C. cruenta Sacc.
Other scientific names
Pseudocercospora cruenta (Sacc.) Deighton (teleomorph Mycosphaerella cruenta Latham)
C. phaseoli Dearness et Bartholomew
C. caracallae (Speg.) Chupp.
Significance
Cercospora leaf spot and blotch of beans are caused by Cercospora canescens Ellis Martin and C. cruenta Sacc. There are no reports of serious losses in Latin America, although defoliation has occurred in Colombia [Schwartz, 1989].
Symptoms
Symptoms include brown or rust-colored lesions which may coalescence and vary in shape and size. C. canescens produces irregularly shaped, light brown lesions with a gray center in leaves, pods, stems, and branches. These lesions may have a grayish center with slightly reddish border. Lesion may dry and portions fall out, leaving a ragged appearance. Premature defoliation may occur, but vigorously growing leaves but seldom infect the trifoliolates. Blemish may occur on steams and pods and the fungi can become seed-borne. A pink to purple discoloration occurred on bean seed with C. kikuchii [Schwartz, 1989].
 Cercospora canescens (photo: Agriqua.doae.go, 2009.) |
Hosts
Cercospora canescens has been reported in common bean, lima bean, cowpea, mung bean, hyacitinth bean, peanut, soybean, and Centrosema spp. Although the pathogen has been reported on many tropical legumes [Lenné, 1994].
Geographic distribution
Those fungi occur in Brazil, Colombia, Puerto Rico, Trinidad, Jamaica, Venezuela, Argentina, and USA but can be serious in the Philippines [Schwartz, 1989].
Biology and transmission
High relative humidity favors infection and disease development. Epidemic development depends on prolonged periods of high humidity. Spreads occurs mainly through raindrop splash [Lenné, 1994].
Detection/indexing method at CIAT
- Blotter test method [Kameswara et al., 2006] and mycological characterization throughout stereoscopy and optical microscopy.
Treatment/control
- Copper fungicides applied to foliage are effective against Cercospora leaf blotch [Schwartz, 1989].
- The seed treatment with Imidacloprid (0.1%) for 2 h and Carbendazim (50 WP) + Thiram (75WP) at 2 g/kg seed followed by foliar sprays of Imidacloprid (0.02%) and Carbendazim (0.05%) for 30 and 45 days has controlled cercospora leaf spots in Cowpea [Dubey, 2006].
Procedure followed at the centers in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
References and further reading
Agriqua.doae.go. 2009. http://agriqua.doae.go.th/plantclinic/clinic/plant/mungbean
CAB International. 2007. http://www.cabicompendium.org/cpc/home.asp. Accessed on July 2009.
Dubey SC. 2006. Integrated management of cercospora leaf spots and yellow mosaic of urdbean (Vigna mungo). Indian Journal of Agricultural Sciences 76 (8):485-489.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Lenné JM. 1994. Diseases of Centrosema. In: Lenné JM, Trutmann P, editors. Diseases of tropical pasture plants. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI), Oxon, GB. p. 43-60.
Schwartz HF. 1989. Additional fungal pathogens. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 231-259.
Diaporthe pod blight, Phomopsis seed decay, Stem canker, Stem blight
Scientific name
Phomopsis phaseoli (Desm.) Sacc. (teleomorph: Diaporthe phaseolorum (Cooke et Ellis) Sacc.)
Significance
Diaporthe pod blight of beans is caused by the fungus Phomopsis phaseoli (Desm.) Sacc. No estimates of its prevalence or importance are currently available, although has been reported that is a weak parasite in Honduras [Schwartz, 1989].
Symptoms
Symptoms appear first on leaves as irregularly shaped brown lesions surrounded by a distinct border. Black pycnidia and, occasionally, perithecia form in a zone or are scattered throughout the lesions. Pods infections may then occur and pods become discolored with pycnidia present in lesions [Schwartz, 1989].
Hosts
Phaseolus spp., Phaseolus lunatus, Ipomoea, Allium, Arachis, Capsicum, Hibiscus esculentus, Lupinus, Lespedeza, Strophostyles, Vigna [Punithalingam, 1972], Soybean (Glycine max) [Asante, 1998], Centrosema spp. [Lenné, 1994].
Geographic distribution
Cosmopolitan
Biology and transmission
D. phaseorolum produces hyaline, oblong ascospores measuring 10-12 by 2-4 µm and having one septation [Schwartz, 1989]. Diaporthe has an ostiolate perithecium, fromed in clusters in a stroma composted of both host and fungal tissue, with the outher margin of the stroma delimited by a broad blackened zone. Perithecia in groups or sometimes separate, subglobose to obpyriform, oblique to lateral, with ostiolar nacks convering and erumpent through outer tissues of host [Hanlin, 1990]. The fungus can be seed-borne in soybeans and in beans [Schwartz, 1989].
Detection/indexing method in place at the CGIAR Center at CIAT
- Blotter test method [Kameswara et al., 2006; ISTA, 2008] and mycological characterization throughout stereoscopy and optical microscopy.
Procedure in case of positive test
- Rejection of accession and health monitoring in the field during the new process of multiplication of species.
Protocols at other organizations
International Rules for Seed Testingg (ISTA). 2008. Annexe to Chapter 7: Seed Health Methods: 7-016: Acidified PDA method for the detection of Phomopsis complex on Glycine max.
References and further reading
Asante SNA, Wolffhechel H, de Neergaard E. 1998. First Report of Diaporthe phaseolorum var. meridionalis on Soybean Seeds from Ghana. Plant Disease 82 (12):1401.
Hanlin RT. 1990. Illustrated genera of Ascomycetes. APS Press, St. Paul, MN, USA. 263 p.
Indexfungorum. 2006. www.indexfungorum.org. Accessed on July 2009.
Kameswara RN, Hanson J, Dulloo EM, Ghosh K, Nowell D, Larinde M. 2006. Manual of seed handling in genebanks. Chapter 5 Seed quality. 50-82 p.
Lenné JM. 1994. Diseases of Centrosema. In: Lenné JM, Trutmann P, editors. Diseases of tropical pasture plants. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI), Oxon, GB. p. 43-60.
Punithalingam E. 1972. Diaporthe phaseolorum. [Descriptions of Fungi and Bacteria]. (34):Sheet 336.
Schwartz HF. 1989. Additional fungal pathogens. 2. ed. In: Schwartz HF, Pastor-Corrales MA, editors. Bean production problems in the tropics. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 231-259.
More Articles...
- Nematodes - common bean
- Best practices for safe transfer of common bean germplasm
- Viruses - common bean
- Insects - common bean
- Weeds (common bean)
- Safe transfer of forage legume germplasm
- Import/export of forage legume germplasm
- Guidelines for the safe transfer of forage legume germplasm
- Bacteria - forage legume
- Fungi - forage legume
Subcategories
-
main
- Article Count:
- 11
-
Stog
- Article Count:
- 2
-
Stog-rice
- Article Count:
- 7
-
Stog-sorghum
- Article Count:
- 11
-
Stog-common-bean
- Article Count:
- 10
-
stog-forage-legume
- Article Count:
- 10
-
stog-forage-grass
- Article Count:
- 11
-
stog-maize
- Article Count:
- 9
-
stog-chickpea
- Article Count:
- 10
-
stog-millets
- Article Count:
- 12
-
stog-barley
- Article Count:
- 10
-
stog-groundnut
- Article Count:
- 9
-
stog-pigeon-pea
- Article Count:
- 8
-
stog-wheat
- Article Count:
- 10
-
stog-lentil
- Article Count:
- 9
-
stog-cowpea
- Article Count:
- 10
-
stog-faba-bean
- Article Count:
- 9
-
risk management
- Article Count:
- 4
-
decision support tool
- Article Count:
- 3
-
stog-clonal
- Article Count:
- 23
-
developing strategies
- Article Count:
- 4




