CGKB News and events stog-wheat
Safe transfer of wheat germplasm
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin). ICARDA, Aleppo, Syria (Siham Asaad, Abdulrahman Moukahal).
CIMMYT, as one of the 15 CGIAR centers, has the mandate for care and maintenance of wheat germplasm. The Wellhausen-Anderson Genetic Resources Center provides secure, long-term storage for critical wheat genetic resources; facilitating their use to solve practical breeding problems; improving knowledge about genetic diversity; developing and assessing complementary strategies for in situ and ex situ conservation; exploring genetic diversity at the molecular level; helping develop global databases on wheat genetic resources.
The Plant Genetic Resource Center’s specially designed vaults currently hold 168,000 Triticeae samples, including bread wheat, durum wheat, and triticale (a man-made crop developed by crossing wheat with rye), with significant collections of primitive and wild relatives of wheat. ICARDA also maintains a germplasm collection of wheat and wheat relatives.
Both CIMMYT and ICARDA have responsibilities for wheat and the information on quarantine regulations and guidelines is presented for both institutions.
Information is included on:
- Import and export requirements.
- Technical guidelines for the safe movement of germplasm and detection of relevant pathogens and pests.
- Best practices in place at CIMMYT.
References and further reading
Compendium of Wheat Diseases and Pests. 2010. Edited by William W. Bockus, Robert L. Bowden, Robert M. Hunger, Wendell L.
Morrill, Timothy D. Murray, and Richard W. Smiley. APS Press, St. Paul, MN. USA. ISBN 978-89054-385-6.
Import/export of wheat germplasm
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
It is very important to remember that an International Phytosanitary Certificate is always required for any plant germplasm exchange.
The requirements of countries that received seed from CIMMYT Headquarters located in Mexico, have been collected from the permits granted to CIMMYT for exportation of wheat experimental seed. The information in this table was updated to September 2011.
- The information received from the same country may vary from a permit to another; therefore the latest has been considered the valid one.
- It is advisable before sending a shipment to contact the consignee in the recipient country to confirm the information reported in this table.
Guidelines for the safe transfer of wheat germplasm
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin): ICARDA, Aleppo, Syria (Siham Asaad, Abdulrahman Moukahal).
Technical Guidelines for the Safe Transfer of Germplasm and the Protection of CGIAR Germplasm Banks
Pathogens of quarantine significance of wheat tested by the Seed Health Laboratory of CIMMYT and ICARDA
|
Viruses
|
|
Barley Stripe Mosaic Virus
|
|
Wheat Streak Mosaic Virus
|
|
Bacteria
|
|
Xanthomonas translucens pv. undulosa
|
|
Pseudomonas syringae pv. atrofaciens
|
|
Fungi
|
|
Alternaria triticina
|
|
Claviceps purpurea
|
|
Cochliobolus sativus
|
|
Cochliobolus spicifer
|
|
Cochliobolus victoriae
|
|
Gibberella zeae
|
|
Hymenula cerealis
|
|
Magnaporthe oryzae Triticum patotype
|
|
Monographella nivalis
|
|
Phaeosphaeria nodorum
|
|
Pyrenophora tritici-repentis
|
|
Sclerophthora macrospora
|
|
Tilletia controversa
|
|
Tilletia indica
|
|
Tilletia tritici
|
|
Tilletia laevis
|
|
Urocystis agropyri
|
|
Ustilago tritici
|
|
Insects
|
|
Trogoderma granarium
|
|
Nematodes
|
|
Anguina tritici
|
|
Weeds
|
|
Cirsium arvense
|
Bacteria - wheat
Contributors to this page: CIMMYT, Mexico (Etienne Duveiller, Monica Mezzalama, Eloise Phipps, Thomas Payne, Jesper Norgaard), Independent consultant (Jesse Dubin).
|
Contents: |
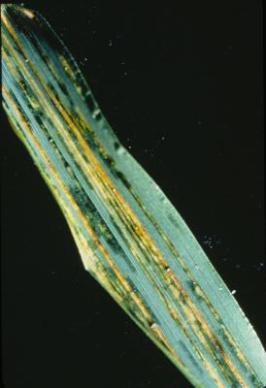
Black chaff; Bacterial leaf stripe; Bacterial leaf streak
|
Bacterial black chaff occurs primarily on the
Bacterial stripe occurs primarily on the
As seen here, droplets of sticky yellowish exudate may appear with extended periods of rain or dew. (photo: CIMMYT) |
Pathogen name
Xanthomonas translucens pv. undulosa (Smith, Jones & Reddy 1919) Vauterin, Hoste, Kersters & Swings 1995.
Other scientific names
Xanthomonas campestris pv. undulosa.
Importance
High phytosanitary importance; present in Mexico and Syria.
Significance
The pathogen is distributed worldwide. Black chaff on wheat is caused by X. translucens. The species X. translucens encompasses different pathovars. Each of these pathovars has a narrow host range, sometimes overlapping. Wheat is mainly affected by X. translucens pv. undulosa and, occasionally by X. translucens pv. cerealis. Yield losses are difficult to quantify precisely; estimates range from negligible to more than 40%. The disease tends to appear more frequently in breeding stations than farmers fields.
Symptoms
Black blotches occur on wheat glumes (hence the name black chaff). Leaf streak or stripe symptoms, of water-soaked lesions in the form of streaks or spots, appear on leaves, awns, and peduncles. These lead to tan or translucent necrotic lesions on leaves and awns and purple to black lesions on peduncles, occasionally with yellow centers.
The translucent water-soaked stripes characteristic of early leaf streak produce abundant droplets of honey-like exudate under humid conditions. If undisturbed, these harden into yellowish granules that stud the surface of the lesion and are easily detachable. With dew or rain the droplets coalesce to form conspicuous milky drops that may spread over the leaf surface and dry as a thin, grayish, almost transparent film or flakes. The alternating bands of healthy and infected tissue on awns produce a ‘barber pole’ appearance, a useful diagnostic feature in the field.
Symptoms on wheat glumes are similar to a non-infectious condition known as pseudo-black chaff or melanosis, caused by expression of the Sr2 gene. Symptoms on glumes may also resemble those caused by Phaeosphaeria nodorum in regions where both diseases occur. Hence, diagnosis must include isolation of the pathogen.
Hosts
Wheat, barley, rye, triticale.
Geographic distribution
Widely distributed, occurring in many of the principal cereal-producing countries of the world. Some confusion exists due to problems with identification and taxonomy of closely related pathovars.
EPPO distribution map (NB map refers to Xanthomonas translucens pv. translucens, conflating the undulosa and campestris pathovars): http://www.eppo.org/
Biology and transmission
Seedborne externally and internally. Also spread by rain-splash, plant contact, and insects, with infection through stomata or wounds. Requires free moisture for infection and spread. Survives on crop debris, gramineous weeds and healthy leaves.
Detection/indexing methods used
- At CIMMYT: Isolation on Wilbrink’s medium, Immunofluorescence test, CIMMYT procedures based on Duveiller et al. (1997).
- At ICARDA: Xts Agar test, IN test
Treatment/control
- Seed soaking in hot, acidified cupric acetate will eradicate the pathogen, but the treatment can be phytotoxic. Heat treatment has been found to be effective.
Procedures followed at the centers in case of positive test
- At CIMMYT: Seed for genebank storage and exportation is destroyed.
- At ICARDA: Heat treatment 75°C for 5 days, Valuable genebank seed are regenerated under controlled conditions
References and further reading
CIMMYT. Wheat Doctor Information sheet: Bacterial stripe. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Date accessed 06 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). pp. 17-18.
Duveiller E. 1990. A seed detection method of Xanthomonas campestris pv. undulosa, using a modification of Wilbrink’s agar medium. Parasitica 46: 3-17.
Duveiller E, Bragard C. 1992. Comparison of immunofluorescence and two assays for detection of Xanthomonas campestris pv. undulosa in seeds of small grains. Plant Disease 76: 999-1003.
Duveiller E, Fucikovsky L, Rudolph K. (eds.). 1997. The Bacterial Diseases of Wheat: Concepts and Methods of Disease Management. Mexico, D.F.: CIMMYT.
EPPO. Data Sheets on Quarantine Pests: Xanthomonas translucens pv. translucens. [online] Available from URL: http://www.eppo.org/ Date accessed 06th April 2010
Maes M, Garbeva P. 1995. Development of a PCR-based detection method for Xanthomonas campestris pv. translucens. Bulletin OEPP/EPPO Bulletin 25: 203-209.
Maraite H, Bragard C, Duveiller E. 2007. The status of bacterial diseases of wheat. Keynote presented at the 7th International Wheat Conference, 27 November–2 December, Mar del Plata, Argentina. In H.T. Buck, J.E. Nisi, and N. Salomon (eds.), Wheat Production in Stressed Environments. Dordrecht, The Netherlands: Springer. pp. 37-49.
Mamluk OF, Al-Ahmed, M, Makki MA. 1990. Current status of wheat diseases in Syria. Phytopathologia Medit. 29: 143-150.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 56-57. [online] Available from URL: http://www.cimmyt.org/ Date accessed 07 April 2010
Stromberg KD, Kinkel LL, Leonard KL. 2004. Quantifying the effect of bacterial antagonists on the relationship between phyllospere population sizes of Xanthomonas translucens pv. translucens and subsequent bacterial leaf streak severity on wheat seedlings. Biological Control 29: 58-65.
Basal glume rot, Bacterial glume rot
|
On the spikelets, lesions generally start
Lesions may also develop on the kernels |
Pathogen name
Pseudomonas syringae pv. atrofaciens (McCulloch 1920) Young, Dye & Wilkie 1978
Other scientific names
Pseudomonas atrofaciens
Importance
High.
Significance
Sporadic weak pathogen. Yield losses due to poor grain fill have been reported.
Symptoms
Infections begin as small, water-soaked lesions, dark green turning brown to blackish in color, that tend to be inconspicuous on unripe wheat heads. On the spikelets, lesions appear at the base of the glumes, resulting in darkened or streaked tissues that are more conspicuous on the inner surface; these may spread over the entire glume. Diseased kernels have a faint brown to black discoloration at their base, and in advanced stages are shrunken and stained brown-black at their embryo end. Under humid conditions a whitish gray bacterial ooze may occur. Infection may also appear in the stem (with dark discoloration) or leaves (with small, irregular lesions).
Hosts
Wheat, barley, oats, rye.
Geographic distribution
Distribution is worldwide but incidence and reports are rare.
Biology and transmission
Seedborne. Also survives on crop debris and various grass hosts, and is disseminated by insects and rain splash.
Detection/indexing methods used
- At CIMMYT: Seed washing and dilution plating on King’s medium B followed by physiological test procedures for identification of Pseudomonas pathovars (Duveiller et al. 1997).
- At ICARDA: King’s medium B test
Treatment/control
- There is no known treatment to eradicate the pathogen.
Procedures followed at the centers in case of positive test
- At CIMMYT: The seed lot is destroyed (the pathogen is quarantined in Mexico).
- At ICARDA: Not applicable
Other protocols
Immunofluorescence: Pseudomonas syringae pv atrofaciens Fluoriscan-IF™ Neogen Ltd, UK, ADGEN, Phytodiagnostics.
References and further reading
Alexandrova M, Zaccardelli M, Stefani E, Bazzi CC. 1995.Testing for Pseudomonas syringae pv. atrofaciens and Xanthomonas campestris pathovars on cereals in Italy. Bulletin OEPP/EPPO Bulletin 23: 437-448.
CIMMYT. Wheat Doctor information sheet: Basal glume rot. [online] Available from URL: http://wheatdoctor.cimmyt.org/ Date accessed 07 April 2010
Diekmann M, Putter CAJ. (eds.). 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14: Small Grain Temperate Cereals. Rome: Food and Agriculture Organization of the United Nations (FAO) and International Plant Genetic Resources Institute (IPGRI). p.16.
Duveiller E, Fucikovsky L, Rudolph K. (eds.). 1997. The Bacterial Diseases of Wheat: Concepts and Methods of Disease Management. Mexico, D.F.: CIMMYT. [online] Available from URL: http://www.cimmyt.org/Research/ Date accessed 06 April 2010
Fessehaie A. 1993. Über die Isolation des Erregers der basalen Spelzenfäule an Getreide, Pseudomonas syringae pv.atrofaciens ((McCull.) Young, Dye, Wilkie) aus Gerste, Hafer und Wildgräsern. Diploma thesis, Institut für Pflanzenpathologie und Pflanzenschutz, Universität Göttingen.
Mavridis A, Meyer D, Mielke H, Steinkampf G. 1991. Zum Auftreten und zur Schadwirkung der basalen Spelzenfäule beim Sommerweizen. KaliBriefe (Büntehof) 20: 469-473.
Prescott JM, Burnett PA, Saari EE, Ransom J, Bowman J, de Milliano W, Singh RP, Bekele G. 1986. Wheat Diseases and Pests: A Guide for Field Identification. Mexico, D.F.: CIMMYT. pp. 58-59. [online] Available from URL: http://www.cimmyt.org/ Date accessed 07 April 2010
Vassilev V, Lavermicocea P, DiGiorgio D, Iacobellis N. 1997. Syringomycins and syringopeptins in the basal glume rot of wheat incited by Pseudomonas syringae pv. atrofaciens. In K. Rudolph, T.J. Burr, J.W. Mansfield, D. Stead, A. Vivian, and J. von Kietzell (eds.), Pseudomonas syringae Pathovars and Related Pathogens. Dordrecht, The Netherlands: Kluwer Academic Publishers. pp. 210-214.
von Kietzell J, Rudolph K. 1997. Epiphytic occurrence of Pseudomonas syringae pv. atrofaciens. In K. Rudolph, T.J. Burr, J.W. Mansfield, D. Stead, A. Vivian, and J. von Kietzell (eds.), Pseudomonas syringae Pathovars and Related Pathogens. Dordrecht, The Netherlands: Kluwer Academic Publishers. pp. 29-34.
von Kietzell J, Rudolph K. 1991. Variation in virulence of strains of Pseudomonas syringae pv. atrofaciens causing basal glume rot of cereals. In: Proceedings of the 4th International Work Group on Pseudomonas syringae pathovars. Florence, Italy. pp. 117-123.



 stog-wheat
stog-wheat