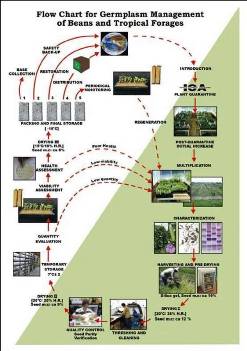
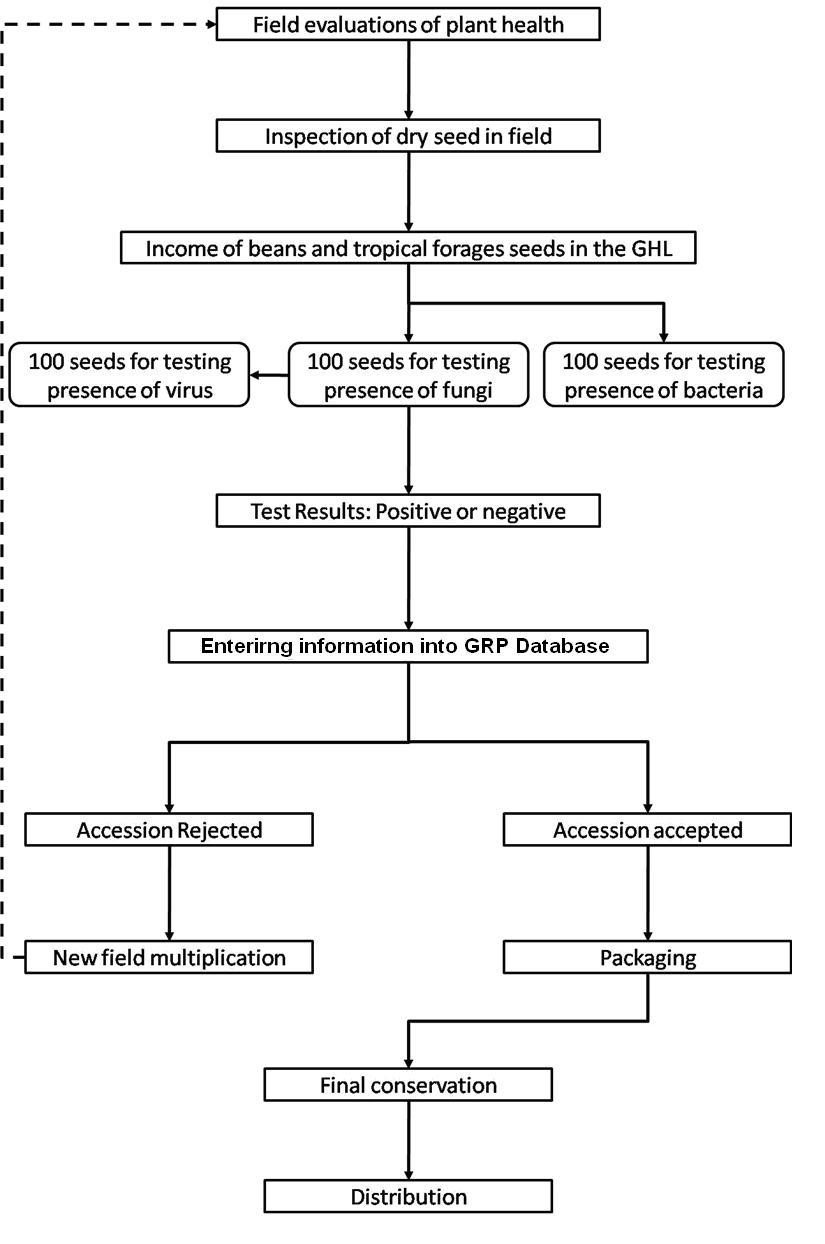
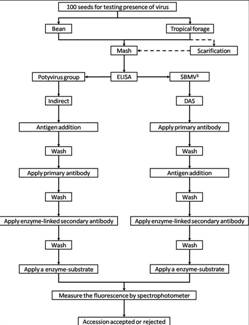
CGKB News and events Management strategies
Bacteria - forage legume
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell); ICARDA, Syria (Siham Asaad).
|
Contents: |
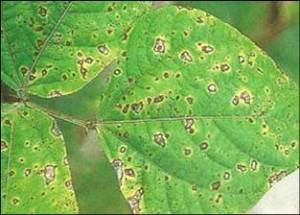
Common blight, fuscous blight (English)
|
Common blight, fuscous blight (photo: http://photos.eppo.org/index.php/image/ |
Scientific names
Xanthomonas axonopodis pv. phaseoli (Smith) Vauterin et al
Other scientific names
Xanthomonas campestris pv. Phaseoli (Smith) Dye Xanthomonas phaseoli var. fuscans (Burkholder) Starr & Burkholder
Significance
Important (spread and loss of yields).
Symptoms
On leaves: initially small water soaked lesions develop narrow, yellow halos. Lesions may enlarge and coalesce, causing extensive necrosis. Lesions may also occur on stems and pods. Infected seeds are sometimes wrinkled and the hilum may be discoloured. Symptoms similar to halo-blight of bean.
On seed: If the infection occurred when the pods were young, the seed may rot or be variously wrinkled and shrivelled. If the bacteria enter by way of the funiculus, only the hilum may be discoloured, but this is difficult to detect on dark-seeded varieties. Strains producing the brown pigment (so-called fuscans strains) give more conspicuous seed discoloration.
Hosts
The principal host is Phaseolus vulgaris and Phaseolus lunatus but other legume species are naturally infected, including P. lunatus, Vigna aconitifolia, V. radiata, and Vigna umbellate. Lablab purpureus and Mucuna deeringiana are possibly natural hosts. P. coccineus, P. acutifolius and Lupinus polyphyllus are hosts only by artificial inoculation (Bradbury, 1986). Macroptilium lathyroides.
Geographic distribution
EPPO region: Found in Egypt, Finland (unconfirmed), Lithuania, Moldova, Morocco (unconfirmed), Norway, Poland (unconfirmed), Sweden (unconfirmed). Widespread in Bulgaria, Hungary, Lebanon and Spain; locally established in France, Germany, Greece, Italy, Netherlands, Portugal (Madeira), Romania, Russia (European), Slovakia, Slovenia, Switzerland, Turkey and Yugoslavia. Found in the past but not established in the Czech Republic, Israel.
Asia: Bangladesh, Brunei Darussalam, Cambodia, China (Heilongjiang, Henan, Hunan, Jilin, Jiangsu, Liaoning, Zhejiang), Cyprus, Georgia, Hong Kong, India (Delhi, Maharashtra, Rajasthan, Uttar Pradesh), Indonesia, Israel, Japan, Lebanon, Korea Democratic People's Republic, Korea Republic, Malaysia, Myanmar (Burma), Nepal, Philippines, Sri Lanka, Taiwan, Thailand, Turkey, United Arab Emirates (IMI, 1996), Viet Nam, Yemen.
Africa: Angola, Burundi, Central African Republic, Egypt, Ethiopia, Kenya, Lesotho, Madagascar, Malawi, Mauritius, Morocco, Mozambique, Nigeria, Rwanda, Somalia, South Africa, Sudan, Swaziland, Tanzania, Tunisia, Uganda, Zaire, Zambia, Zimbabwe.
North America: Bermuda, Canada (Ontario), Mexico, USA (more prevalent east of the Rocky Mountains: Colorado, Hawaii, Michigan, Montana, Nebraska, New York, Texas, Wisconsin, Wyoming).
Central America and Caribbean: Widespread in Central America; Barbados, Costa Rica, Cuba, Dominica, Dominican Republic, El Salvador, Guatemala, Honduras, Jamaica, Martinique, Nicaragua, Panama, Puerto Rico, St. Vincent and Grenadines, Trinidad and Tobago.
South America: Argentina, Brazil (widespread), Chile, Colombia, Ecuador, Paraguay, Uruguay, Venezuela.
Oceania: Australia (New South Wales, Queensland, Victoria, Western Australia), New Zealand, Samoa.
EU: Present.
VeryVery widespread (Anonymous, 1971).
Biology and transmission
The bacterium enters the leaves via stomata or wounds, and subsequently invades the intercellular spaces, causing a gradual dissolution of the middle lamella. The stem is entered in three ways: via the stomata of the hypocotyl and epicotyl; through the vascular system of the leaf; or from infected cotyledons. The seed is penetrated via the vascular system of the pedicel and funiculus. The micropyle also serves as a point of entry into the seed. Direct penetration of seed has not been observed. The pathogen either remains in the seedcoat or passes to the cotyledons when the seed germinates, and so infection of the young plant results.
The bacterium can remain viable for several years beneath the seedcoat.
The disease is severe under conditions of high rainfall and humidity, with maximum development around 28°C. Dissemination in the field occurs in wind-driven rain, and insects.
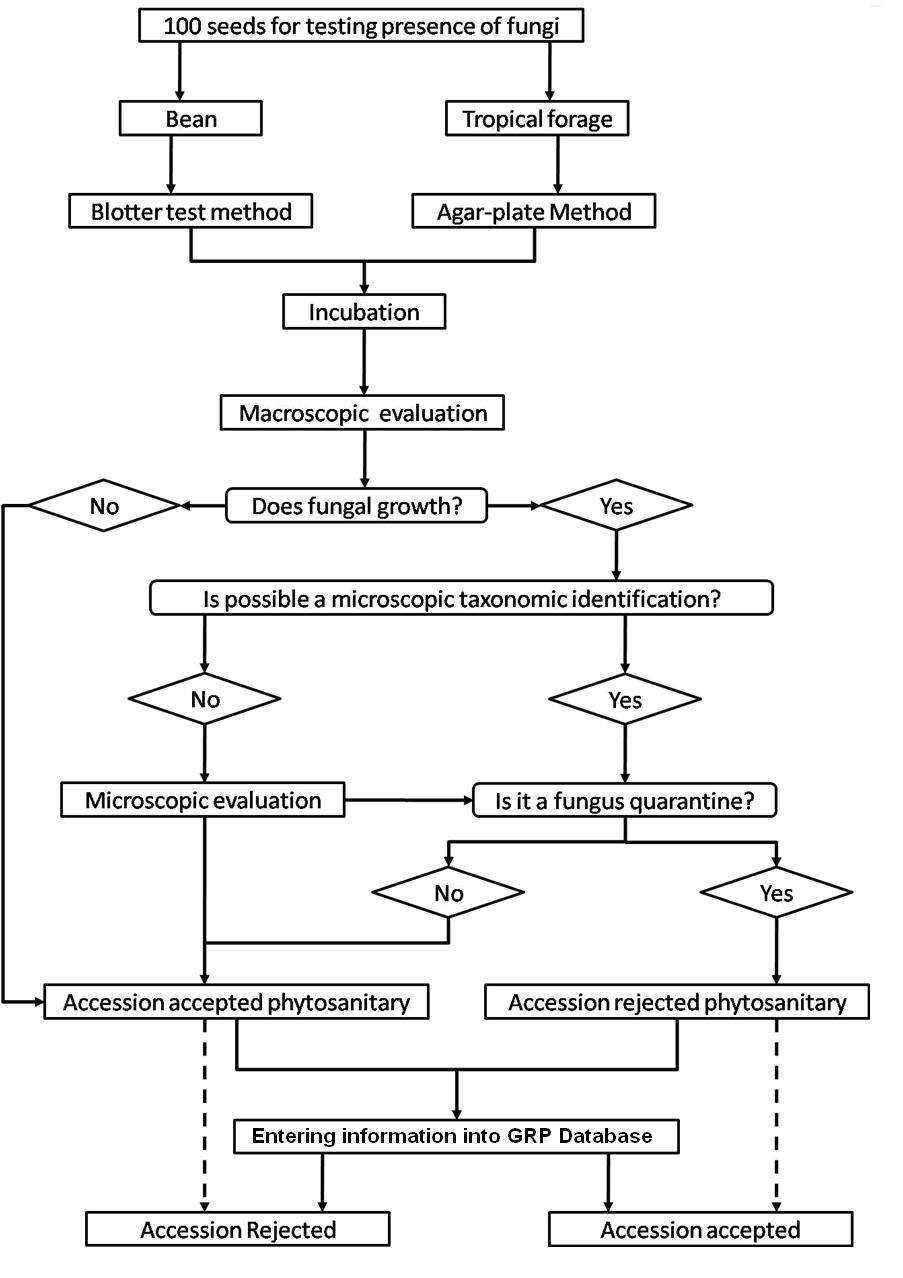
Detection/indexing method
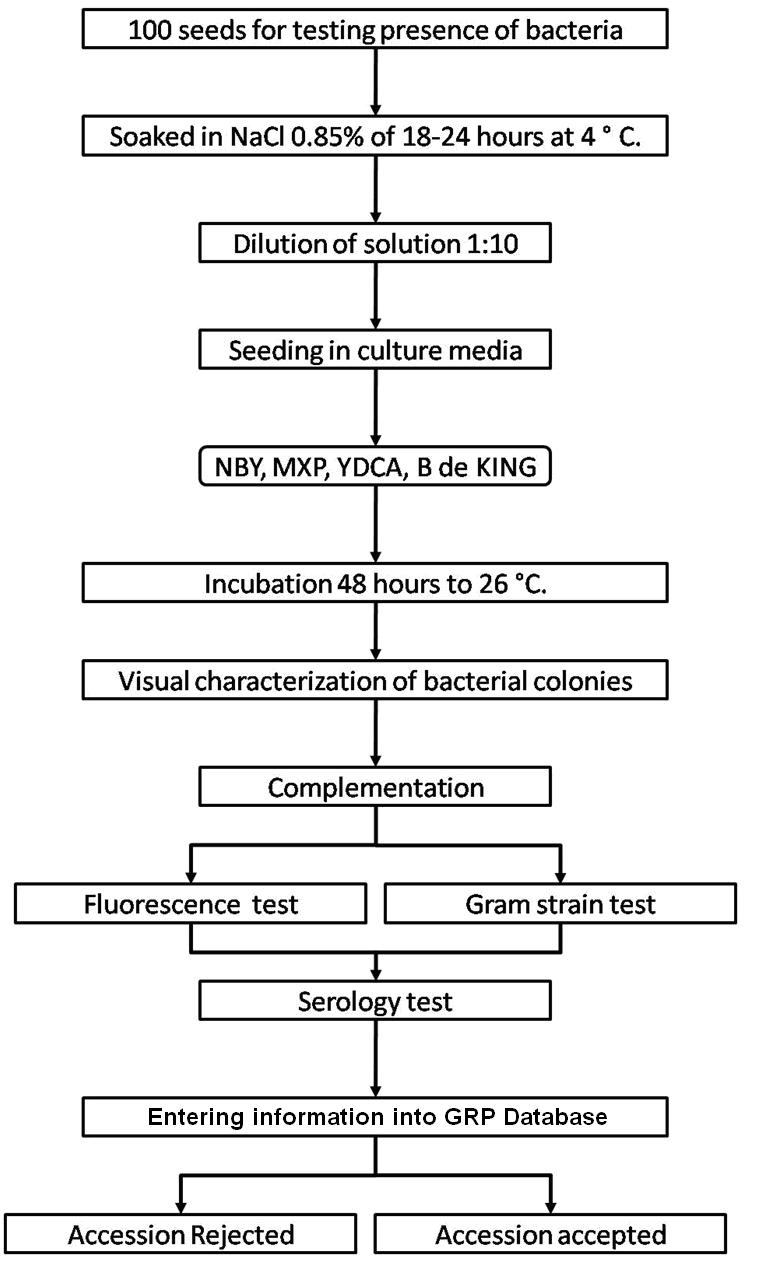
- At CIAT: Agar plate dilution technique. MXP (Claflin et al 1987) and YDCA semiselective culture medium and serology.
Treatment/control
- Procedure followed in case of positive test
References of protocols at EPPO, NAPPO or other similar organization
OEPP/EPPO Data sheets on quarantine organisms. Prepared by CABI and EPPO for the EU under Contract 90/399003
References and further reading
Anonymous. 1971. CMI distribution maps of plant diseases. No. 401 (edition 2).
Commonwealth Agricultural Bureaux, Slough.
Bradbury JF. (1986) Guide to plant pathogenic bacteria. CAB International, Wallingford, UK.
Claflin LE, Vidaver AK, Sasser M. (1987) MXP, a semiselective medium for Xanthomonas campestris pv. phaseoli. Phytopathology 77, 730-734.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
|
Photo by Howard F. Schwartz, Colorado State University, Bugwood.org |
Scientific names
Pseudomonas syringae pv. phaseolicola
Other scientific names
Pseudomonas savastanoi pv. phaseolicola
Significance
Important (spread and loss of yields).
Symptoms
Symptoms first appear as dark, water-soaked leaf spots, up to 3 mm in diameter and sometimes surrounded by a broad lemon-coloured halo. No lesions have been observed on stems or leaf stalks. When these pathogenic bacteria enter the leaves through wounds or natural openings like stomates they multiply rapidly and induce the formation of lesions. The lesions, which are watersoaked at first, soon become brown and dry and are usually surrounded by a yellow halo. When many lesions grow together, large areas of dead leaf tissue may develop. The halo is actually caused by a toxin that is produced by the bacteria within the lesion. As the toxin diffuses out into the leaf tissue it causes breakdown of chlorophyll and creates the halo. When toxin from infected leaves is translocated to the growing point of the shoot, the new leaves that develop are often stunted and chlorotic.
Hosts
Cajanus cajan, Lablab purpureus, Macroptilium spp., Phaseolus coccineus, P. lunatus, P.vulgaris, Pueraria spp., Vigna angularis, V. radiata, Neonotonia wightii. Isolates of the pathovar are categorised into three races on the basis of the reactions of a range of differential bean cultivars.
Geographic distribution
Worldwide (Anonymous, 1973). In temperate climatic conditions and in the tropics at medium to high altitudes (1000-2500 m). Race 3 of the pathogen has been found only in East and Central Africa.
Biology and transmission
Cell of the pathogen are single, straight rods and move by multitrichous polar flagellar. They are Gram-negative and strictly aerobic. The optimal growth temperature is 20 - 23o and on agar the bacterium produces white to cream coloured colonies wich exhibit a bluish tinge and often a green fluorescent pigment. P. syringae pv. phaseolicola survives in infected seeds and in vegetable(plant) residues in the surface of the soil until the environmental conditions are propitious for the development of the infection.
Detection/indexing method
- At CIAT: Agar plate dilution technique. King B semiselective culture medium and serology.
Treatment/control
- Procedure followed in case of positive test
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM, Trutman P. 1990. Diseases of Tropical Pasture Plants. CAB Interantional in association with Natural Resource Institute and CIAT.
Lascano CE, Spain JM. Establecimiy Renovación de Pasturas. Red Interacional de Evaluación de Pastos Tropicales. Veracruz, Mexico 1988
Schwartz HF. 1989. Halo blight In: Schwartz, H.F. and Pastor Corrales, M.A. Bean Production Problems in the tropics. CIAT. Cali, Colombia, pp 285-302
Taylor JD. 1970. The quantitative estimation of the infection of bean seed with Pseudomonas phaseolicola (Burkh.) Dowson. Ann appl. Biol. 66: 29-36.
Bacterial blight, Bacterial Pod Rot
Scientific names
Pseudomonas fluorescens biotipo II Migula
Other scientific names
Bacillus fluorescens Trevisan 1889
Bacterium fluorescens (Trevisan 1889) Lehmann and Neumann 1896
Liquidomonas fluorescens (Trevisan 1889) Orla-Jensen 1909
Bacillus fluorescens Flgge 1886
Symptoms
The disease is characterizates by water-soaked lesions on young growth especially young leaves, petioles and terminals, progressing to bliht, necrosis, dieback and defoliation. Necroticspots develop on older leaves and flowering and seed productions may be severely reduced.
Hosts
Centrosema sp. (C. acutifolium, C. pubescens, C. brasilianum, C. macrocarpum, C. schiedeanum and C. virginianum), Allium, Brassica, Phaseolus sp., Solanum spp., Leucaena leucocephala.
Geographic distribution
Colombia and Costa Rica.
Biology and transmission
Although the species is normally considered to be a saprophyte, Biotipe II causes root rots and leaf blights on a range of hostd, including legumes. It is distinguished from other fluorescent pseudomonas by the following characterisitic: more than one polar flagellum, no poyocyanin or carotenoid pigments produced, no growth at 41o C, gelatin hydrolysed but not starch and utilization of a wide range of carbon sources. Disease is favoured by high relative humidity and moderately high temperatures. The bacterium can survive unfavourable periods on affected plants and soil for as long as 6 weeks. The bacterium is seed-borne and levels of infections as high as 32 % have been found in seed lots of C. acutifolium.
Detection/indexing method
- At CIAT: Agar plate dilution technique. King B semiselective culture medium.
Treatment/control
- Procedure followed in case of positive test
References and further reading
Arias B, Lenne JM. 1988. Sistemas de produccion de Pasturas Tropicales de semillas de Centrosema acutifolium y efecto de bacterioocidas en la incidencia de Pseudomonas fluorescens Biotipo II. Pasturas Tropicales Vol. 10, 11-18.
Bradbury JF. 1986. Guide to plant pathogenic Bacteria, CAB Interantional, Wallingford, UK
Guevara Gómez CL, Lenné JM, Torres GC. 1983. Etiology of dieback of Centrosema spp. and effect of the pathogen on yield and quality during the period of establishment of the legume. Phytopathology 73, 122
Lenné JM, Torres GC, Victoria JI. 1981. Bacterial leaf spot and dieback of Centrosema spp. Proc. Fifth Int. Conf. Plant Path. Bact Cali, 35-38.
 |
 |
Scientific names
Curtobacterium flaccumfasciens pv. flaccumfaciens (Hedges) Collins & Jones
Other names
Bacterial wilt (Phaseolus beans), bacterial tan spot (soyabeans)
Symptoms
Seedlings are stunted, wilted and usually die. In some plants the affected parts are dull green in colour and sometimes breaking of the stems. Infected pods show discoloured sutures and may show yellowish areas.
Foliar interveinal chlorosis and necrosis. When the lower stem and root are cut longitudinally and observed, the vascular system is often discolored brown to black. The younger the plant becomes infected, the more severe the damage to the plant. Seedlings are frequently severely stunted or killed. If plants survive to maturity, seeds may show yellow or purple discoloration.
Seeds of white-seeded cultivars, when infected systemically, are bright-yellow; in cultivars with coloured seed coats, the coloration is less conspicuous. There may be a little yellow slime at the hilum, and seeds may be variously shrivelled. The colour mutants, aurantiacum and violaceum, produce an orange and purple discoloration, respectively, in the seedcoat.
Hosts
Lablab purpureus, Phaseolus coccineus, Phaseolus lunatus, Phaseolus vulgaris, Vigna angularis, Vigna unguiculata, Zornia spp. and possibly Glycine max.
All members of Leguminosae.
Geographic distribution
Bacterial wilt of beans has been reported in North and South America, Europe, and Australia
EPPO region: Recorded in Albania, Ukraine. Found but not established in Greece and Hungary; locally established in Bulgaria (unconfirmed), Romania, Tunisia, Turkey (unconfirmed), Russia (Far East, Southern Russia; only on soyabean) and Yugoslavia. Reports from Belgium (OEPP/EPPO, 1982), France, Germany and Switzerland have not been substantiated.
Asia: Russia (Far East), Turkey (unconfirmed).
Africa: Mauritius, Tunisia.
North America: Canada (Ontario), Mexico (unconfirmed), USA (first reported in 1920, especially in irrigated high plains and Midwest, but not reported since early 1970s except in Iowa on soyabeans; Hall, 1991. Specific records from Colorado, Connecticut, Iowa, Idaho, Michigan, Montana, Nebraska, Ohio, Oregon, Virginia, Wisconsin).
South America: Colombia, Venezuela.
Oceania: Australia (New South Wales, Queensland, South Australia, Victoria).
Europe: Present (Belgium, Bulgaria, Greece, Hungary, Romania, Yugoslavia.
Biology and transmission
Seed transmitted externally or internally in Phaseolus vulgaris and possibly in Glycine max. The pathogen can survive from 5 to 24 years in seeds.
Seed is the most important means of survival and spread. The bacteria can overwinter on weeds or in crop debris in the field. In the field it has been known to survive in soil for at least two winters between bean crops rotated with wheat. There are no reports of vectors, but the nematode Meloidogyne incognita may assist entry by providing wounds.
C. flaccumfaciens pv. flaccumfaciens can infect in the absence of rain; it has not been observed to enter via stomata. Once within the plant, the bacterium colonizes the vascular tissue.
Detection/indexing method
- At CIAT: Agar plate dilution technique. NBY (Nutrient Broth Yeast extract) semiselective culture medium and serology.
Treatment/control
- Procedure followed at the centers in case of positive test
References of protocols at EPPO, NAPPO or other similar organization
OEPP/EPPO (1982) Data sheets on quarantine organisms No. 48, Corynebacterium flaccumfaciens. Bulletin OEPP/EPPO Bulletin 12 (1).
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Hedges F. (1926) Bacterial wilt of beans (Bacterium flaccumfaciens Hedges), including comparisons with Bacterium phaseoli. Phytopathology 16, 1-22.
Torres GC, Lenné JM, Victoria JI. Bacterial wilt of Zornia spp. caused by Corynebacterium flaccumfaciens. Conference paper Proceedings of the Fifth International Conference on Plant Pathogenic Bacteria. 1982 pp. 74-79.
Fungi - forage legume
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell); ICARDA, Syria (Siham Asaad).
Alternaria leaf spot; Alternaria blight; Bean leaf blight
 |
 |
Scientific name
Alternaria alternata (Fr.) Keissler
Other scientific name
Alternaria tenuis (Nees)
Symptoms
Spots are formed by concentric rings of dark tissue.
Symptoms first appear at the tips of leaflets as small pale spots on margins. These progress into the leaflets and become necrotic and dark brown to blackish in appearance. The infection spreads to other branches and the leaf infection gives the plant a blighted appearance.
Leaf spot, fruit rot, blossom rot, blight. Opportunistic pathogen on numerous hosts resulting in leaf spot, fruit rot, blight, bulb rot, blossom rot.
Hosts
The fungus has a wide host range and thus can survive throughout the year. Desmodium spp and Stylosanthes spp. Multiple genre in multiple families
Geographic distribution
Cosmopolitan
Biology and transmission
Prevent spread of the disease by a single foliar spray of dithane M-45 (3 L/ha).
Alternaria alternata is a ubiquitous fungus that can be found on many kinds of plants, animals, and other substrata, including foodstuffs and soil. Many strains of this fungus are known to be saprophytic, but some are notorious for causing severe diseases.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- At ILRI: Culture on oatmeal agar, isolation and identification under microscopy
- At ICARDA: Media test
Treatment/control
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration process started in field.
References and further reading
http://www.icarda.org/Publications/
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Desmodium. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 61-76. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Lenné JM. (1994) Diseases of Stylosanthes. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 21-42. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Tu JC. 1983. Efficacy of iprodione against alternaria black pod and white mold of white beans. Canadian Journal of Plant Pathology 5(2):133-135.
Zillinsky FJ. 1983. Common diseases of small grain cereals: a guide to identification. International Maize and Wheat Improvement Center, Mexico. 141 p.
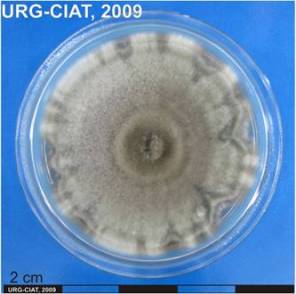
Ascochyta leaf spot or blotch; Leaf spot (or blotch) in beans
 |
 |
Scientific name
Ascochyta spp.
Ascochyta phaseolorum Sacc
Other scientific name
Phoma exigua Desmaz. var. exigua
Significance
Ascochyta leaf spot is a minor, but widely distributed disease, usually producing leaf spots on the lower leaves on wheat, oat, triticale, barley and numerous grasses.
Symptoms
Pycnidial fungus infects leaves, stems and roots.
Young leaf spots are irregularly circular with grey to brown centres surrounded by a border of light green-yellow tissue. Other spots are light to dark brown and frequently zonate, marginal, terminal or discrete, cracking in the centre when the dead tissue finally drops out, 3 to 5 cm diameter. Lesions common on pulvini result in considerable defoliation.
Pycnidia are formed within leaf spots. The fungus cause leaf blight, blotch and streak or stripe. Lesions develop as yellowish brown to grey-colored elongated spot, spreading down the leaf to the tip and subsequently affecting whole leaves.
Hosts
Canavalia ensiformis, Glycine max, Lablab (Dolichos) niger, Phaseolus acutifolius, P. atropurpureus, P. aureus, P. calcaratus, P. lathyroides, P. limensis, P. lunatus, P. mungo, P. nanus, P. richardianus, P. trilobus, P. vulgaris, Vigna catjang', V. coerulea, V. sesquipedalis, V. sinensis, V. unguiculata, Voandezia subterranea. Also by inoculation on Althaea rosea, Cannabis sativa, Capsicum annuum, Fagopyrum sagittatum, Gossypium spp., Hibiscus esculentus, Lycopersicon esculentum, Malva verticillata, Nicotiana tabacum, Solanum melongena, Vicia sativa (Crossan, 1958; Zherbele, 1958).
Ascochyta phaseolorum causes target-spot lesions on several genera in the Leguminosae.
Wide host range: vegetable crops, weeds, indigenous species (Australia)
Andropogon spp., Macroptilium atropurpureum, Paspalum spp.
Geographic distribution
Africa (Congo, Ethiopia, Kenya, Malawi, Rhodesia, Sudan, South Africa, Tanzania, Uganda, Zambia); Asia (China, India, Japan, Malaya, North Borneo, Pakistan, Sarawak), Australasia & Oceania (New Guinea, Solomon Is.); Europe (Denmark, Great Britain, Czechoslovakia, Netherlands, Sweden, U.S.S.R.); North America (U.S.A.); South America (Peru), Australia
Africa, Asia, Australia, New Zealand, the Caribbean, Pacific islands and South America.
Biology and transmission
Wound parasite and appears to be ubiquitous soil-borne organism.
Seed borne, persisting in a viable condition for 24 years. Viability and virulence also unimpaired after storage in culture at 1°C or 10-12°C for 20-25 months. The pathogen may spread by infected haulms and by spores disseminated by splashing rain drops from infected seedlings. Also spread in pods of harvested plants stored in stooks in the field in cool, moist, weather.
Conidia are spread by raindrop splash and the fungus has been isolated from seed. It is possible that the fungus may survive in roots, crowns and infected seed. Ascochyta leaf spot is common in areas having mild, temperature climates. The fungus persist on the dead leaves of native grasses.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- At ILRI: Freezing blotter method, PDA
- At ICARDA: Malt Excract Agar, PDA
Treatment/control
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration process started in field.
References and further reading
http://www.cababstractsplus.org/
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Kaiser WJ, Hannan RM. 1987. Seed-treatment fungicides for control of seedborne Ascochyta lentis on lentil. Plant Disease 71:58-62.
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 77-96. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Lenné JM. (1994) Diseases of other pasture grasses. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 169-194. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Zillinsky FJ. 1983. Common diseases of small grain cereals: a guide to identification. International Maize and Wheat Improvement Center, Mexico. 141 p.
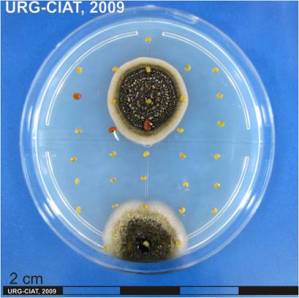
Botrytis gray mold; Botrytis head blight; Stem rot by Botrytis cinerea.
 |
|
www.microscopy-uk.org.uk/mag/imgfeb08/Feb-Ima... |
Scientific name
Botrytis cinerea Pers. 1794
Teleomorph. Sclerotinia fuckeliana (de Bary) Fuckel
Significance
Minor economic importance.
Symptoms
Dark grey fungal growth on growing tips, flowers and pods.
Infected flowers drop thus reducing pod set.
can affect leaves, stems, crowns, flowers, flower buds, seeds, seedlings, bulbs, and just about any other part of a plant with the exception of the roots.
Lesions develop as brown, water-soaked areas becoming greyish on drying in the inflorescence with extensive blossom blighting and apical dieback. Under humid conditions, profuse sporulation occurs on inflorescences and stems. Buff-coloured stem lesions develop where diseases flower parts lodge.
Hosts
Cajanus cajan, Cicer arietinum
Stylosanthes spp., it has been found on S. guianensis, S. hamata, S. humilis and S. viscosa.
Geographic distribution
Syria, Morocco, USA.
Colombia and Zimbabwe.
Biology and transmission
The shed flowers and leaves on the ground are covered with sporulating mycelium of the fungus.
It survives on infected debris and is better at 10oC.
It survives and remains ineffective up to 8 months in the soil.
Cultural control includes deep summer ploughing and reduction of plant density and increase in air passage between the plants.
Chemical control: use of Trichoderma spp. as bio-control agents.
Conidia are mainly airborne and may also be carried by raindrop splash. Diseases inflorescences, on which sporulation is profuse under wet conditions, are important sources of inoculum in epidemics. The fungus survives as sclerotia or as mycelium associated with host lesions.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- At ILRI: PDA culture and identification by microscopy.
- At ICARDA: PDA.
- At ICRISAT: Blotter test.
Treatment/control
Procedure followed at the centers in case of positive test
- Rejected of accession and new regeneration seed process in field.
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
La Mondia JA, Douglas SM. 1997. "Sensitivity of Botrytis cinerea from Connecticut Greenhouses to Benzimidazole and Dicarboximide Fungicides." Plant Disease 81(7): 729.
Lenné JM. (1994) Diseases of Aeschynomene. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 97-107. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI: Oxon, GB.
Lenné JM. (1994) Diseases of Stylosanthes. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 21-42. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Yigal E, Jürgen K, Fokkema NJ. 1994. Control of infection and sporulation of Botrytis cinerea on bean and tomato by saprophytic yeasts. Phytopathology (USA) 84(10):1193-1200.
Zalewski JC, Johnson ER. 1977. Benalate tolerance in gray mold beans. Proc. Oerg. Hortic. Soc. 68: 95-97. Cited in: Johnson, K.B., Powelson, M.L. 1983. Analysis of spore dispersal gradients of Botrytis cinerea and gray mold disease gradients in snap beans. Phytopathology 73(5):741-746.
Cercospora leaf spot and blotch; Cercospora leaf and stem spot; Leaf spots caused by Cercospora and related genera
|
agriqua.doae.go.th/.../mungbean/image/spot.jpg |
Scientific name
Cercospora canescens Ellis & G. Martin
Other scientific name
Cercospora aeschynomenes A.S. Mull. & Chupp, Cercospora arachidicola S. Hori (teleomorph Mycosphaerella arachidis Deighton), Cercospora canescens Ellis & G. Martin Synonym: Mycosphaerella cruenta Latham, Cercospora centrosematis Chupp & A.S. Mull., Cercospora commonsii Sacc., Cercospora cylindrospora F. Stevens & Solheim, Cercospora desmodii Ellis & Kellerm. Current name: Passalora desmodii (Ellis & Kellerm.) U. Braun, Cercospora desmodiicola G.F. Atk., Cercospora fusimaculans G.F. Atk. Current name: Passalora fusimaculans (G.F. Atk.) U. Braun & Crous, in Crous & Braun, Cercospora leucaenae-leucocephalae Raghu Ram & Mallaiah, Cercospora melaleuca Ellis & Everh, Cercospora stylosanthis Ellis & Everh., Passalora stylosanthis (Speg.) U. Braun.
Significance
Minor
Symptoms
Leaf spot, fruit spot.
On clovers, other than sweet clovers, the leaf spots range from light brown to almost purplish-black. The spots on affected leaves are usually rectangular and lie between the veins of the leaf. When dew is on the leaves, the lesions appear silvery from the many spores produce by the fungus. Sunken areas may nearly cover the stems of diseased plants. As spot on the leaves enlarge or merge, the affected areas shrivel and turn brown and the leaves either drop off or prematurely die. On sweet clover and alfalfa, large, circular, ashy-grey spots develop on older leaves, which later shrivel and drop. On first-year stems of clover, reddish-brown lesions sometimes develop in the fall after frost. The disease becomes noticeable on second-year sweet clover when plants began to blossom. Occasionally the fungus is found early on shoots that are dying back after cutting or grazing. The fungus also attacks the flower heads, causing maturing seed to fall. Disease seeds maybe shriveled and discolored, or they may show no visible sign of infection.
The fungus causes brown to black, circular to angular leaf lesions with chlorotic haloes which expand, especially under humid conditions, causing chlorosis, necrosis and premature defoliation. Sporulation is common on the leaf undersurface. The fungus may affect pods and seed of grain legumes.
Hosts
Dolichos lablab, Lablab niger, Phaseolus spp., Vigna radiata, Vigna unguiculata, Vigna and Voandzeia subterranea (Vigna subterranea), Trifolium spp., Medicago sativa.
Arachis spp., Aeschynomene spp., Centrosema spp., Desmodium spp., Macroptilium atropurpureum, Stylosanthes spp.
Geographic distribution
Widespread in warm regions
USA, Colombia
Central and South America as well as in Australia, Barbados, Malaysia, Philippines, Puerto Rico and Sudan.
Biology and transmission
Plant damage is dependent of weather conditions, summer black stem is always present, and frequently causes extensive leaf shriveling and premature defoliation.
The fungus persists on old stems; it produces fruiting structures abundantly during warm wet weather. Spores are disseminated by wind or rain.
The principal source of initial inoculum is probably conidia produced on groundnut crop residues in the soil. Inoculum is blown or splashed on to leaves giving rise to primary infection. Conidia are disseminated by wind, rain splash and insects leading to secondary infection. The pathogen may also survive on volunteer groundnut plants and on groundkeepers. Long distance spread may be by movement of infected crop debris, pods or seeds externally contaminated with conidia. There is no evidence of the disease being internally seed-borne. The role of seed-borne inoculum on disease spread is not known. High relative humidity and moderately high temperatures favor infection and disease development. Spread occurs mainly through raindrop splash.
Detection/indexing method in place at the CGIAR Center
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy
- At ILRI: Culture on oatmeal agar, isolation and identification under microscope
Treatment/control
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
http://www.cababstractsplus.org/
Culbreath AK, Stevenson KL, Brenneman TB. 2002. Management of Late Leaf Spot of Peanut with Benomyl and Chlorothalonil: A Study in Preserving Fungicide Utility. Plant Disease 86 (4):349.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 77-96. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Stylosanthes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 21-42. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Anthracnose; Leaf and pod anthracnose
Scientific name
Colletotrichum gloeosporioides (Penz.)Penz. & Sacc.
(teleomorph Glomerella cingulata (Stonem.) Spauld. and Schrenk.)
Significance
Anthracnose is the most widely distributed and damaging disease of various forages throughout the tropics. Anthracnose reduces dry-matter production with associated reduction in nutritive value and seed losses.
Symptoms
Seedlings that emerge from infected seed can develop lesions on the root, hypocotyl or cotyledons. Lesions are generally oval shaped, pink to beige and up to 2 cm long. These cause the stem to bend and may progress to infect the pods and seeds.
Leaf and petiole lesions are 1-3 mm in diameter with cream to light grey centers and dark margins and elliptical stem lesions 2-6 mm in length and similar in colour to leaf lesions. Acervuli are generally visible in lesions. Under humid warm conditions, leaf and petiole lesions coalesce, causing defoliation, while stems lesions develop into cankers and girdle stems.
Hosts
Aeschynomene falcata, Desmodium barbatum, Stylosanthes scabra, Stylosanthes guianensis, Stylosanthes hamata, Stylosanthes spp.
Arachis spp., Aeschynomene spp., Calopogonium spp., Cassia spp., Centrosema spp., Desmodium spp., Leucaena spp., Macroptilium spp., Pueraria spp. and Stylosanthes spp.
Infects a wide variety of hosts
Geographic distribution
Cosmopolitan
South America (Colombia), Australia
Anthracnose is widely distributed throughout India, Central and South America and the Caribbean, being recorded from Barbados, Brazil, Colombia, Costa Rica, Ecuador, Peru, Venezuela, Pacific and subtropical USA and China.
Biology and transmission
Anthracnose is largely a seed-borne disease, but may be spread on trash, machinery or by animals and birds.
Lesions produce an abundance of spores that are spread through the crop by rain-splash. Anthracnose can survive over summer on crop residue. Spores are subsequently spread by rain-splash to infect newly emerged seedlings.
Severe disease developed on plants incubated at 20-30°C and 24 hours of leaf wetness after inoculation. Spread occurs mainly through raindrop splash. The fungus is Seedborne. Seed transmission is believed to be responsible for the rapid spread of anthracnose in northern Australia and probably for this global movement.
Detection/indexing method in place at the CGIAR Center
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy
- At ILRI: Blotter method, culture on oatmeal agar, isolation and identification with microscope
Treatment/control
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome
Lenné JM, Trutmann P. (1994) 'Diseases of tropical pasture plants.' (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Aeschynomene. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 97-107. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Leucaena. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 108-128. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 77-96. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Stylosanthes. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 21-42. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Weeds PL, Chakraborty S, Fernandes CD, d'A. Charchar MJ, Ramesh CR, Kexian Y, Kelemu S. 2003. Genetic Diversity in Colletotrichum gloeosporioides from Stylosanthes spp. at Centers of Origin and Utilization. Phytopathology 93 (2):176.
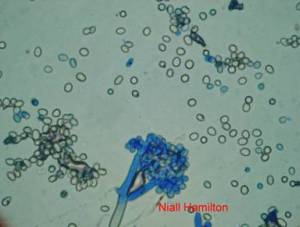
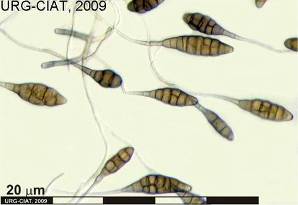
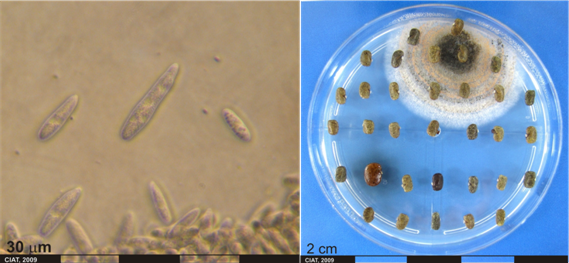 Conidia and culture of Colletotrichum gloeosporioides (Penz.) (photos:CIAT) |
Anthracnose; Soybean anthracnose
Scientific name
Colletotrichum truncatum (Schwein.) Andrus & W.D. Moore 1935
Other scientific name
Colletotrichum dematium f. truncatum, Vermicularia truncata
Significance
Important on many species
Symptoms
When infected seeds are planted pre-emergence and post-emergence damping-off may occur. Sunken, dark brown lesions develop on the cotyledons of seedlings. Seedling lesions may expand to the stem and kill young plants.
Plants may become infected at any stage of development but chances of infection tend to increase with maturity. The most common symptoms are brown, irregularly shaped spots on stem, pods and petioles. The girdling of petioles by large lesions results in premature defoliation. When pods are infected, mycelium may completely fill the cavity and no seeds are produced (pod blanking) or fewer and/or smaller seed form. Seed that does form may appear brown, moldy and shriveled or may look normal. Dark acervuli develop in lesions on all host tissue areas. Leaf infections result in leaf rolling, necrosis of laminar veins, petiole cankers and premature defoliation. This disease is commonly observed on soybean stems at maturity.
Dark irregular lesions occur on leaves, stems and pods which are covered by small black dots.
Colletotrichum truncatum produces similar macroscopic symptoms to those caused by C. gloeosporioides (leaf and stem lesions with cream to light grey centers and dark margins and elliptical stem lesions and similar in colour to leaf lesions) but is a much less serious that this pathogen. It is often a secondary colonizer of lesions caused by the latter.
Hosts
Glycine max, lentil
Arachis spp., Aeschynomene spp., Calopogonium spp., Cassia spp., Centrosema spp., Desmodium spp., Leucaena spp., Macroptilium spp., Pueraria spp. and Stylosanthes spp.
Geographic distribution
USA , Australia
This disease is distributed across Central and South America and the Caribbean, being recorded from Barbados, Brazil, Colombia, Costa Rica, Ecuador, Peru, Venezuela, Pacific and subtropical USA and India.
Biology and transmission
The fungus produces crowded, black acervuli on infected tissues. This will be dark bodies looking much like “pin cushions” on the plant surface when viewed with 20X or greater magnification. Acervuli produce many spores (conidia) which is how the disease spreads. The fungus overwinters in infested crop debris and has been shown to be seed-borne.
Anthracnose infections typically occur during warm, moist weather. Infection typically occurs when plant leaf wetness, rain or dew periods exceed 12 h per day.
Anthracnose is a common secondary disease and management is not typically warranted. Crop rotation and crop residue incorporation will reduce inoculum by breaking down infested residue.
Seed treatment fungicides should be used on seed planted from fields with high levels of anthracnose. Foliar fungicide applications have been shown to be an effective way or reducing late season anthracnose.
Anthracnose development is favored by prolonged high relativity humidity and moderate high temperatures. Spread occurs mainly trough raindrop splash.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- At ILRI: Culture on oatmeal agar, isolation and identification under microscope
Treatment/control
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
http://pdc.unl.edu/agriculturecrops/soybean/anthracnose
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome
Lenné JM, Trutmann P. (1994) 'Diseases of tropical pasture plants.' (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Aeschynomene. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 97-107. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Leucaena. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 108-128. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 77-96. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Stylosanthes. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 21-42. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
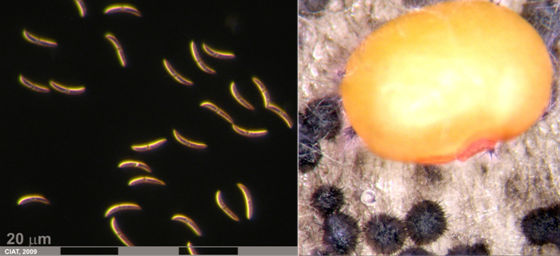 Conidia and culture of Colletotrichum truncatum (Schwein) (photos:CIAT) |
Curvularia leaf spot; Curvularia blight
Scientific name
Curvularia spp.
Curvularia lunata (Wakker) Boedijn 1933 Synonym: Cochliobolus lunatus R.R. Nelson & Haasis 1964
Curvularia verruculosa Tandon & Bilgami Synonym: Pseudocochliobolus verruculosus Tsuda & Ueyama
Curvularia trifolii Kauffman Boedijn 1933
Symptoms
Infected leaves are distinquished by the presence of large, yellowed area that turns watery grey and translucent and then light brown. A yellowish band usually outlines the advancing edge of the area. In some cases, diseased areas that originate at a leaf tip become v-shaped. Sometimes the dead, v-shaped part of the leaf curls down. The fungus can invade the entire leaflet and grow down the petiole, causing wilt and killing the leaves. The fungus does not attack stolons.
Fungal disease that causes spots on leaves. The lesions are brown, short-stripe shaped, 1 to 2 mm long and the damage is severe.
Hosts
Trifolium spp., Trifolium repens
Centrosema spp., Desmodium spp., Stylosanthes spp., Zoysia matrella, Z. japonica, Sorghum halepense, Brachiaria platyphylla, Setaria glauca, Cynodon dactylon
Geographic distribution
Japan, China, USA
Has been recorded in Australia, Florida, North Carolina, Malaysia, Colombia, Trinidad
Biology and transmission
The fungus is spread by wind-borne spores. The disease develops more rapidly during warm, wet weather; temperatures of 75oF to 78oF are most favourable.
Since the disease occurs mainly on leaves, grazing or clipping the plant then the disease is first observed will remove much of the infected tissue. This in turn reduces the potential to infect new growth.
Disease activity is most common during spring and summer when temperatures are between 21 and 30°C. Affected leaves initially exhibit small, chocolate brown spots, followed by dieback of leaves from the tips, and eventually blighting of entire tillers. Symptoms appear in small, irregular patches as much as 15 cm in diameter, but numerous patches may coalesce to impact large sections of turf. Infected turf appears tan or brown from a distance, but often turns black during periods of wet or humid weather.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- At ILRI: Culture on oatmeal agar, isolation and identification under microscope
- At ICARDA: PDA
Treatment/control
Procedure followed at the centers in case of positive test
- Rejected of accession and new regeneration seed process in field.
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Stylosanthes. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 21-42. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Pratt RG. 2006. Johnsongrass, Yellow Foxtail, and Broadleaf Signalgrass as New Hosts for Six Species of Bipolaris, Curvularia, and Exserohilum Pathogenic to Bermudagrass. Plant Disease 90 (4):528.
Prithiviraj B, Singh UP, Manickam M, Srivastava JS, Ray AB. 1997. Antifúngical activity of bergenin, a constituent of Flueggea microcarpa. Plant Pathology 46:224-228.
Roberts JA, Tredway LP. 2008. First Report of Curvularia Blight of Zoysiagrass Caused by Curvularia lunata in the United States. Plant Disease 92 (1):173.
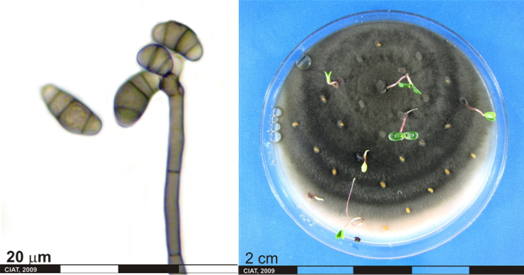 Conidia and culture of Curvularia sp. (photos:CIAT) |
Fusarium wilt; Fusarium root rot
Scientific name
Fusarium oxysprorum Schlecht. Emend. Snyd. & Hans.
Other scientific name
Fusarium lateritium f. ciceris, Fusarium merismoides f. ciceris, Fusarium orthoceras var. ciceris
Significance
Important for seedline survival in genebank operations
Symptoms
The pathogen causes vascular wilt in chickpea. Wilting can occur in seedling or adult stages. The initial symptom is drooping of petioles and rachis along with leaflets. Within 2 to 3 days, drooping is seen on the entire plant. Roots of wilted plants show no external rotting, but when split vertically, clearly show internal discoloration of the xylem.
The disease can affect the crop at any stage.
In legumes the symptoms include wilting of leaves and shoots, chlorosis of lower leaves, defoliation, internal root browning and death. Older plants are more tolerant.
Hosts
Cicer arietinum, Cajanus cajan, Lens culinaris.
Pigeon pea, pea and lentil are symptomless carriers of the pathogen.
Leucaena leucocephala.
Geographic distribution
Algeria, Bangladesh, Chile, Ethiopia, India, Iran, Italy, Lebanon, Malawi, Mexico, Morocco, Myanmar (Burma), Pakistan, Peru, Spain, Sudan, Syria, Tunisia, USA.
Biology and transmission
The disease is transmitted to new areas through infected seed (chlamydospore-like structures in the hilum region of the seed). Soil-borne inoculum is also a source of primary infection. Once it is introduced to soil, it is difficult to eradicate the pathogen. Therefore it is important to stop spread of the pathogen through seed.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- At ILRI: Blotter method, Culture on oatmeal agar, isolation and identification under microscope
- At ICARDA: Oatmeal Agar, PDA, freezing blotter
- At ICRISAT: Blotter test
Treatment/control
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Leucaena. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 108-128. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
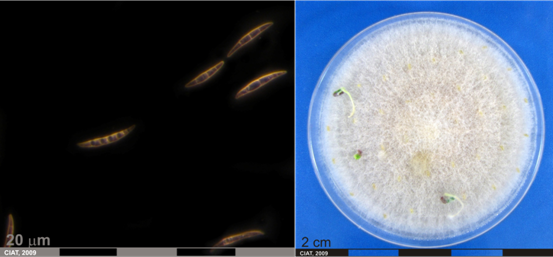 Conidia and culture of Fusarium oxysprorum Schlecht. Emend. Snyd. & Hans. (photos:CIAT) |
Scientific name
Macrophoma sp
Symptoms
Lesion on disease leaves are dark and compose of firmnecrotic tissue. Marginal necrosis occurs along the apical portion of disease leaflets.
Hosts
Geographic distribution
Biology and transmission
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
Treatment/control
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/ IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant
Genetic Resources, Rome.
Dry root rot; Charcoal rot; Charcoal dry root rot; Dry-weather wilt
Scientific names
Macrophomina phaseolina (Tassi) Goid.
Anamoprh: Rhizoctonia bataticola
Other scientific names
Botryodiplodia phaseoli, Dothiorella cajani, Dothiorella phaseoli,
Dothiorella philippinensis, Fusicoccum cajani, Macrophoma cajani,
Macrophoma corchori, Macrophoma phaseoli, Macrophoma phaseolina, Macrophoma sesami, Macrophomina phaseoli, Macrophomina philippinensis,Rhizoctonia lamellifera, Sclerotium bataticola, Tiarosporella phaseoli, Tiarosporella phaseolina
Significance
Important
Symptoms
Seed and seedling rots, root and stem rots, rotting of developing pods and seeds. The tap root turns black and later becomes rotten, shredded and studded with sclerotia. Pods are also attacked, the pathogen rapidly infects the fruits, developing symptoms of blacknuts and leading also to concealed damage. Infected seeds are discoloured, small, shriveled, and have a dirty black appearance. Severely attacked seeds are covered with profuse growth of the mycelium of the fungus on the inner as well as on the outer surfaces of the two cotyledons, and black sclerotia can be observed in the endosperm tissue.
It caused chlorosis, severe wilting and death of more of plants. Microsclerotia were common on blackened roots of dead plants and in associated soil.
Some infected seeds do not show external symptoms.
Hosts
Infects over 500 plant species
Found throughout the world, causing diseases in a large number of crop species Arachis hypogaea, Cicer arietinum, Glycine max, Stylosanthes guianensis var. pauciflora, Stylosanthes capitata, Stylosanthes humilis.
Geographic distribution
AFRICA, Botswana, Cameroon, Central African Republic, Egypt, Ethiopia, Gambia, Ghana, Ivory Coast, Kenya, Libya, Malagasy Republic, Malawi, Mauritius, Morocco, Niger, Mozambique, Nigeria, Senegal, Sierra Leone, South Africa, Sudan, Swaziland, Tanzania, Tunisia, Togo, Uganda, Upper Volta, Zaire, Zambia, Zimbabwe, ASIA, Bangladesh, Brunei, Burma, China, Hong Kong, India, Indonesia, Iran, Iraq, Israel, Japan, Korea, Kuwait, Lebanon, Malaysia (Sabah, Sarawak, Malaya, Selangor), Nepal, Oman, Pakistan, Philippines, South Yemen, Sri Lanka, Syria, Taiwan, Thailand, Turkey, Yemen
AUSTRALASIA & OCEANIA, Australia, Fiji, Papua New Guinea, Solomon Islands, New Zealand (Auckland- N. Island; Blenheim, Nelson - South Island)
EUROPE, Austria, Cyprus, Denmark, France, Greece, Hungary, Italy, Republic of Ireland, Romania, Spain, Switzerland, United Kingdom, USSR, West Germany, Yugoslavia,
NORTH AMERICA, Canada (Ontario), Mexico, USA,
CENTRAL AMERICA & WEST INDIES, Bermuda, Jamaica, Dominica, Puerto Rico, Trinidad, Cuba, El Salvador,
SOUTH AMERICA, Argentina, Brazil, Chile, Colombia, Guyana, Peru, Uruguay, Venezuela.
Biology and transmission
Charcoal rot is both seed-borne and soil-borne. Mycelium in seeds and sclerotia in plant debris in the soil are primary sources of inoculum. The fungus persists in the soil for long periods either as actively growing mycelium or as dormant sclerotia. The pathogen is commonly present in groundnut seeds (mycelium in cotyledons or endosperm) and pods, and can readily be disseminated by their movement. Mycelial fragments as well as sclerotia can be present on the testae of seeds.
Seedling damage can occur when infected seed is planted. Typically, symptoms occur after mid-season during the reproductive stages of crop development. Infected plants produce slightly smaller leaflets than healthy plants and have reduced vigor. As the disease advances, leaflets turn yellow, then wilt and turn brown. The brown leaves remain attached to the petioles. A light gray of silver discoloration will be visible in the taproot and lower stem when plants are split open. Black specs (microsclerotia) will be visible in this tissue of the stem and tap root. Outer tissues will have black, dusty microsclerotia. Plants in the driest parts of the field will typically show symptoms first. Upper pods may have poor fill and general low plant vigor. In some cases, the upper one-third of the plant may have only flat pods without seed.
Microsclerotia (hardened fungal survival bodies) are formed inside infected tissue. These microsclerotia are how the fungus overwinters in infested crop debris and free in soil. Survival of microsclerotia is several years in dry soil but only a few weeks in wet, saturated soils. Infection of soybean typically occurs early in season at emergence and early seedling growth stages. These seedling infections remain latent until environmental stresses (drought and high ambient temperatures) occur during the reproductive and mature pod growth stages.
Charcoal rot thrives in the hottest, driest part of the growing season. This disease can develop at any place where hot, dry conditions exist. Charcoal rot develops when there is a high level of pathogen in the soil and when plants are under stress from hot dry weather.
Plants grown in conditions of high temperatures, drought or poor fertility (too high or too low) are most susceptible.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy
- At ILRI: Blotter method
- At ICARDA: Blotter and PDA
- At ICRISAT: Blotter test
Treatment/control
References and further reading
Frison E A, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Stylosanthes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 21-42. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
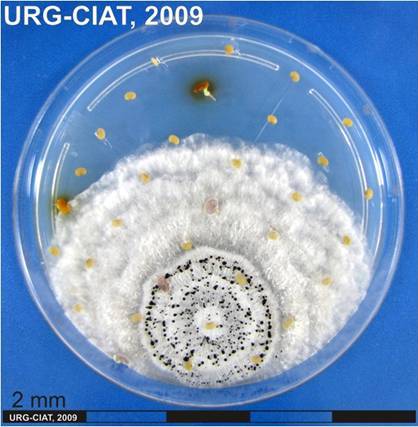
 Culture and fruiting bodies of Macrophomina phaseolina (Tassi) Goid (photos:CIAT) |
Storage fungi (high moisture: 5-8% in peanuts); Seed-borne fungi on dry beans
Scientific names
Penicillium sp.
Penicillium puberulum Bainern1907 Syn: Penicillium aurantiogriseum Dierckx 1901
Penicillium viridicatum Westling 1911 Syn: Penicillium aurantiogriseum var viridicatum (Westling) Frisvad & Filt 1990
Penicillium verruculosum Peyronel 1913
Penicillium citrinum Tom 1910
Penicillium islandicum Sopp 1912
Penicillium urticae Bainer 1907 Syn: Penicillium griseofulvum Dierckx
Significance
Serious parasites of seed primordial, maturing seeds, stored seeds and grains
Symptoms
Deterioration and spoilage of seeds
Hosts
Phaseolus vulgaris, Cajanus cajan, Lens culinaris, Lupinus termis, Arachis hypogaea, Glycine max
Beans and cereals
Geographic distribution
Africa, Asia, Southeast Asia, North America
Croatia, Sudan, Kenya, Philippines, Thailand, Uganda, USA, Canada
Biology and transmission
P. puberulum produces aflatoxin (Fluvocumarinic substance). Aflatoxin is generally produced after harvest when moisture content of the seeds is high.
P. viridicatum produces ochratoxin (Nephrotoxic mycotoxin).
Presence of Penicillium in soil causes contamination in bean seeds.
Detection/indexing method
- At CIAT: Not of significant important
- At ILRI: Blotter method, Culture on oatmeal agar, isolation and identification under microscope
- At ICARDA: Wash test
Treatment/control
Procedure followed at the centers in case of positive test
- Seed disinfection
References and further reading
http://tpa.usab-tm.ro/cercetare/anale/2008/vol/L66_vol%20XIV_2008_Morar_Oana.pdf
http://www.fao.org/docrep/X5036E/x5036E0n.htm
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
 |
 |
Scientific name
Pestalotiopsis sp.
Significance
Pestalotiopsis spp. were formerly regarded as weak, opportunistic pathogens which caused little damage plants.
Symptoms
Initial symptoms on young pods appeared as bright yellow, scattered spots 1 to 2 mm in diameter that later became raised, black, and corky within 12 days. As the disease progressed, the lesions coalesced and formed large cankers, which resulted in unmarketable pods.
Hosts
Desmodium spp., Cassia spp., Psidium guajava, Vicia faba. and diverse fruits and ornamental plants.
Geographic distribution
Worldwide
Biology and transmission
The genus Pestalotiopsis Steyaert is a heterogeneous group of coelomycetous fungi consisting of 205 described species that are differentiated primarily on conidial characteristics such as size, septation, pigmentation, and presence or absence of appendages. However, Pestalotiopsis is a complex genus and can be difficult to classify to the species level because characters such as growth rate, conidial morphology, and fruiting structure characteristics tend to vary within species
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
Treatment/control
Procedure followed at the center in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Hopkins KE, McQuilken MP. 2000. Characteristics of Pestalotiopsis Associated with Hardy Ornamental Plants in the UK. European Journal of Plant Pathology, 106: 77–85.
Keith LM, Velasquez ME, Zee FT. 2006. Identification and Characterization of Pestalotiopsis spp. Causing Scab Disease of Guava, Psidium guajava, in Hawaii. Plant Disease 90 (1):16.
Lenné JM. (1994) Diseases of Centrosema. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 43-60. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Lenné JM. (1994) Diseases of other pasture legumes. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 129-157. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Iboton SI, Devi RKT. 2001. First Report of Broad Bean Canker Caused by Pestalotiopsis disseminata in India. Plant Disease 85 (2):229.
Angular leaf spot of kidney beans (Phaseolus vulgaris)
Scientific name
Phaeoisariopsis griseola (Sacc.) Ferraris
Other scientific name
Isariopsis griseola Sacc.
Significance
Although the effect of angular leaf spot on the yield and quality on cultivars of beans has not been quantified, moderate to severe defoliation is observed under cool conditions. Angular leaf spot could have a considerable effect on animal production.
Symptoms
Reddish brown lesions on leaves with typical angular margins; sporulation under continuous moisture for 24-48 h; circular or irregular spots on stem, petioles, branches and pods
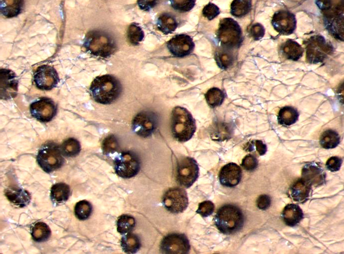
Lower surface of Phaseolus vulgaris leaflet severely infected by Phaeoisariopsis griseola showing coalescence of lesions.
The first sign of the disease is the development of dark, grey-green fungal structures (stromata, synnemata and conidia) on the lower surfaces of leaves under moderate temperature conditions. Development of chlorotic, necrotic, often angular lesions on leaf upper surfaces. Pod lesions (common on beans) have not been reported on legumes.
Hosts
Desmodium cephalotus, Desmodium gangeticum, Desmodium pulchellum, Dolichos lablab, Phaseolus lunatus, Phaseolus multiflorus, Phaseolus vulgaris, Pisum sativum, Vigna unguiculata
Geographic distribution
Widespread: Brazil, Colombia, Costa Rica, Ecuador, Mexico, Panama, Peru, USA (Florida) and Venezuela. The same disease is serious on beans throughout the tropics and especially in East Africa.
Biology and transmission
Transmission through seeds and plant debris. Rain splash and wind help in disease spread.
Seeds are infected at the helium region.
The fungus infects leaf tissue trough stomata and advances intercellularly. Within 9-12 days after infection, intracellular development of the fungus leads to the production of stromata in the substomatal cavity and Sporulation can occur under moist conditions. Moisture is the key factor affecting the development of angular lea spot epidemics and is a prerequisite for infection, synnemata formation and Sporulation. Sporulation is facilitated by dry conditions and spore dispersal by water splash.
Detection/indexing method in place at the CGIAR Center
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy
Treatment/control
- Biological control assays with a protein isolated from seeds of Clitoria tenatea (L.). Conidia were treated with crude protein or sterile water along with a layer of Potato Dextrose Agar. The results showed a fail to germinate after treatment, whereas those treated with sterile water germinated and converted into mycelia
Procedure followed at the center in case of positive test
- Reject accession and new regeneration started in field
References and further reading
2004. Tropical forage pathology . In: Centro Internacional de Agricultura Tropical. Integrated Pest and Disease Management in Major Agroecosystems: Project PE-1: Summary Annual Report 2004 . CIAT, Cali, CO. p. 284-297.
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Kelemu S, Mahuku GS, Segura G. 2005. An antifungal protein of the tropical forage legume Clitoria ternatea controls disease under field and greenhouse conditions [abstract]. Phytopathology (USA) 95(6):S52.
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 77-96. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB. Seed Health General Publication Published by the Center or CGIAR
Ascochyta blight; Black node disease
Scientific name
Phoma exigua Desmaz. var. diversispora (Bubak) Boerema
Other scientific name
P. diversispora Bubak
Symptoms
The leaf spot associated with wet weather blight is characterized by a brown or grey spot usually 1 to 4 mm in diameter (it can be larger in severe cases) surrounded by a red halo. At later stages brown to black specks called pycnidia, which contain the spores of the fungus, may be visible in the lesion. If the leaf spot progresses, cotyledons and young leaves may turn brown and die. The most prominent symptom of the stem canker phase of wet weather blight is often a leaf or leaves that have wilted or died. Below the wilted leaves (sometimes at that node or several nodes lower) is a black canker. As the cankers become older, they become brown with a shredded appearance. Often, the lower part of the plant is relatively healthy, although the lower leaves may have dropped off as a result of the leaf spot phase of the disease.
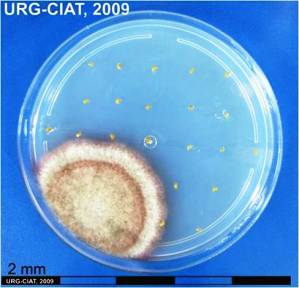
 Fruiting bodies of Phoma exigua Desmaz. var. diversispora (Bubak) Boerema (photo:CIAT) |
Hosts
Beans, Phaseolus vulgaris
Phoma exigua is a fungus that infects numerous species of plants, including common bean, soybean, sunflower, and possibly corn.
Geographic distribution
USA
Biology and transmission
Cool, wet weather is required for development of this disease. Leaf spot caused by P. exigua can contribute to damping-off of seedlings. Typically, dryer and warmer weather will prevent further infections and the disease cycle ends. Wet weather in combination with cool temperatures (night temperatures below 60ºF) can result in infection of the main stem at a node. If cool wet weather persists, the stem may be girdled causing the upper portion of the plant to wilt and die. Infection and spread of the disease is favoured by a combination of factors: the presence of juvenile plant tissue, low temperatures that damage this tissue, and wet weather. Infected stems on older plants may break off easily from the rest of the plant.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy
- At ILRI: Culture on oatmeal agar, isolation and identification under microscope
- At ICARDA: Malt Extract Agar, Freezing blotter method, PDA
Treatment/control
Procedure followed at the centers in case of positive test
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Leaf spots; Black stem spotting
 |
 |
Scientific name
Phoma sorghina (Sacc.) Boerema, Dorenb, and van Kest.
Symptoms
Causes pre-and postemergent damping-off of several legumes. This pathogen is also commonly associated whit necrotic lesions on leaves of Stylosanthes spp. In tropical South America.
Hosts
Desmodium spp., Brachiaria spp., Centrosema spp., Macroptilium atropurpureum, Stylosanthes spp.
Geographic distribution
Nigeria, South America
Biology and transmission
Symptom expression and pycnidial formation required sustained conditions of high relative humidity, so that recognition of the disease may be difficult under dry conditions.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- At ILRI: Oatmeal agar culture and identification with microscope
Treatment/control
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of other pasture grasses. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 169-194. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 77-96. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Stylosanthes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 21-42. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Seed Health General Publication Published by the Center or CGIAR
Pod and stem blight; Leaf spot
Scientific name
Phomopsis sp. (Teleomorph. Diaporthe phaseolorum (Cooke and Ell.) Sacc.)
Symptoms
The most obvious sign of pod and stem blight is the presence of pycnidia on infected material. Pycnidia are small, black fruiting structures which are first seen on the petioles of shed leaves. One of the most definitive diagnostic features of pod and stem blight is the appearance of lines of pycnidia which may cover large sections of the stem or they may occur in clusters near the nodes. Pycnidia can also occur in a scattered arrangement of the pods. Infected seeds may appear healthy or they may be shriveled and cracked. Infected seed are often covered with white mycelium and appear chalky. Several infected seed may not germinate.
Brown, v-shaped areas developed at edges of leaves. The blotches may involve 1/3 or more of the leaf area.
Isolates from Colombia and Brazil caused lesions, as well as, leaf spot and dieback on seedlings of the Centrosema species.
Hosts
Glycine max, Beans, Lucerne, Centrosema spp., Desmodium spp., Macroptilium atropurpureum
Geographic distribution
Throughout most soybean growing areas
Brazil, Colombia, Africa and the Pacific
Southeast Asia
Biology and transmission
The fungus overwinters in infested crop debris and in infected seed. In early summer perithecia are produced which give rise to ascospores for initial infections. Another source of the disease will be conidia produced on debris and infection sites on the plants. Both spore types are splashed onto the plant or distributed with wind. Initial infections are latent until the soybean plant begins to senesce.
While pod and stem blight had little effect on yield, seed quality may be negatively impacted when seed infections occur and Phomosis seed decay develops.
Prolonged periods of warm, wet weather during pod development and maturation favor the spread of the fungus from the pod to the seeds. Very wet conditions will favor stem infections during pod filling stages.
Rotation with corn and other non-legume crops reduces the amount of inoculum available the following season. The incorporation of farm residues in soil provides inoculum since the fungus overwinters in these materials.
Phomosis have been isolated from large, round to irregular, midbrown lesions on leaves of C. macrocarpum and Macroptilium atropurpureum.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy
- At ILRI: Oatmeal agar and indentification through microscope
- At ICARDA: Malt Extract Agar
Treatment/control
- Fungicide Seed Treatment
Procedure followed at the centers in case of positive test
- Depends to the level of inection
- Reject accession and new regeneration started in field.
References and further reading
http://pdc.unl.edu/agriculturecrops/soybean/podandstemblight
http://www.aces.edu/timelyinfo/PlantPathology/2009/January/pp662.pdf
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 77-96. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
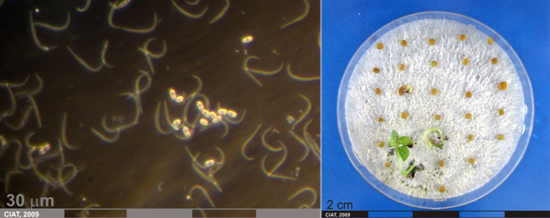 Conidia and culture of Phomopsis sp. (photos:CIAT) |
Rhizoctonia root rot; Rhizoctonia foliar blight
Scientific name
Rhizoctonia solani J.G. Kühn 1858
Other scientific name
Thanatephorus cucumeris (A.B. Frank) Donk 1956
Significance
Minor
Symptoms
Both pre-emergent and post-emergence seedling death can occur with this disease. Pre-emergence symptoms are seed decay and are often not visible in the field. Post-emergence symptoms on seedlings will be the appearance of brown to reddish lesions on stems and roots just below the soil line. These reddish brown lesions may become sunken and girdle the stems and kill the plant. Plants may often appear stunted and unhealthy throughout the season or, less commonly, will die. Often the stand will appear uneven because of stunted plants. On older plants, the pathogen causes a reddish brown dry cortical root rot that may extend into the base of the stem. Later in the season, infections at the base of the plant (cortical rot) may result in plants snapping off during high winds. Root rot can greatly reduce nodulation. Foliar symptoms may include yellowing or wilting of leaves.
Damping-off of legume seedlings; web blight resulting in premature defoliation; leaf rust
Foliar blight appears initially as water-soaked patches in the foliage canopy. Profuse growth of fungal mycelium throughout the foliage causes mats of leaves to be stuck together with mycelia strands. Sclerotia are common on blighted leaves. Under prolonged humidity, the patches may extend, causing considerable rotting and death of foliage. Death leaves and petioles are covered and matted together by greyish brown to white fungal mycelium.
Hosts
Acacia mangium, Acacia auriculiformis, Acacia nilotica, Albizia lebbeck, Glycine max, Faidherbia albida
Albizia spp., , Ravenelia spp.,
Beans, pea; soybean;
Aeschynomene americana, A. histrix, A. brasiliana, Arachis pintoi, A. glabrata, Brachiaria brizantha, B. decumbens, B. dictyoneura, B. humidicola, Calopogonium sp., C. mucunoides, Cassia rotundifolia, Centrosema acutifolium, C. arenarium, C. brasilianum, C. macrocarpum, C. pascuorum, C, plumieri, C. pubescens, C. schiedeanum, C. tetragonolobum, virginianum, Desmodium ovalifolium, D. uncinatum, Desmodium sp., Macroptilium atropurpureum, Neonotonia wightii, Panicum maximum, Pueraria phaseoloides, Stylosanthes capitata, S. guianensis, S. hamata, S. humilis and S. sundiaca.
Geographic distribution
USA
This disease has been recorded in Central and South America, Florida, Malaysia, Papua New Guinea, Solomon Islands, Zambia and globally throughout the tropics.
Biology and transmission
Rhizoctonia solani can be found in most soils and survives as sclerotia (very resistant fungal survival structures) in soil.
Rhizoctonia root rot is usually common in warm, moist sandy soils. Stresses in plants which have been observed to favor disease development include herbicide injury, soil insect damage, hail, sandblasting and soybean cyst nematode feeding.
Damage from Rhizoctonia is commonly observed in areas when there is a long history of soybean production with close rotations or during weather conditions not favourable for seed germination and rapid growth of seedlings.
Initiation and development of foliar blight from foci at the beginning on the wet season are favored by high relative humidity, frequent prolonged rain and moderately high temperatures. In Central and South America, foliar blight is most severe in regions with greater than 1500 mm mean annual precipitation. The fungus probably spreads from perennial plants in the pasture and plants debris in the soil. The pathogen complex is primarily soil-borne. Sclerotia readily survive in soil for several years and are disseminated by wind, rain and animals.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- At ICARDA: PDA
Treatment/control
Procedure followed at the centers in case of positive test
- Crop rotation, fungucid seed treatments
- Reject accession and new regeneration started in field.
References and further reading
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Aeschynomene. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 97-107. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI: Oxon, GB.
Lenné JM. (1994) Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 77-96. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
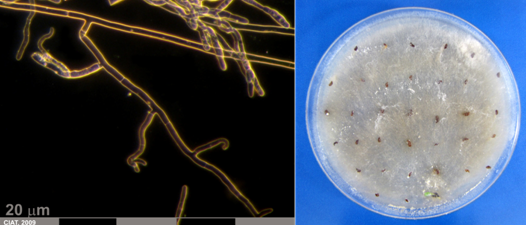 Mycelia and culture of Rhizoctonia solani Kühn (Teleomorph Thanatephorus cucumeris (Frank) Donk.) (photos:CIAT) |
Sclerotium wilt; Collar rot; Inflorescence blight
Scientific name
Sclerotium rolfsii Saccardo (Teleomorph Athelia rolfsii ( Curzi) Tu & Kimbrough
Significance
Minor
Symptoms
Seedlings turn slightly chlorotic before they die.
Affected seedling can easily be uprooted but the lower part of the root usually remains in the soil.
First sign of attack by S. rolfsii is usually partial to complete wilting and collapse of isolated plants. The lower portions of affected plants, especially the collar region and the surrounding soil surface, are often colonized by profuse white mycelia, which differentiates collar rot from other seedling diseases caused by Fusarium, Rhizoctonia or Pythium, and small, brown sclerotia. In Colombia, affected plants usually died. Mature inflorescences are colonized by profuse white mycelium, which develop typical brown sclerotia. Few seed are harvested from affected plants.
Hosts
The fungus is an unspecialized pathogen with extensive host range, as well as, Stylosanthes spp., Centrosema spp., Desmodium spp., Macroptilium atropurpureum, Cassia spp, Cajanus cajan.
Geographic distribution
Australia, Brazil, Colombia, Florida, Malaysia, Panama, Papua New Guinea, Peru, Thailand and Venezuela
Biology and transmission
Usually appears within a month of sowing over field.
Temperatures at about 30 oC and soil moisture at sowing predispose the seedling to infection.
The pathogen finds an excellent substrate in undecomposed stubble.
Cultural control: summer deep ploughing; select well drained fields, keep the fields free of undecomposed organic matter; sow when soil moisture is low; select fields where cereal crops have not been grown in the previous season.
Chemical control: seed treatment with Thiram or captan at 3g/kg or Thiram and Carbendazim (2:1) 0.25%.
Detection/indexing method
- At CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
Treatment/control
Procedure followed at the center in case of positive test
References and further reading
http://jnkvv.nic.in/IPM%20Project/disease-pigeonpea.html
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Lenné JM. (1994) Diseases of Stylosanthes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 21-42. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of Macroptilium atropurpureum. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 77-96. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. (1994) Diseases of other pasture legumes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 129-157. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
 Sclerotia and culture of Sclerotium rolfsii Sacc. (Teleomorph Athelia rolfsii ( Curzi) Tu & Kimbrough (photos:CIAT) |
Nematodes - forage legume
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell); ICARDA, Syria (Siham Asaad).
|
Contents: |
Stem and bulb nematode infection
Scientific name
Ditylenchus dipsaci (Kühn) Filipjev
Significance
A major pest in temperate climates and high-altitude regions of the tropics and subtropics.
Symptoms
Plants become distorted and stunted; infected tissues are spongy; damage can predispose plants to other problems.
Field shows irregular areas of sparse growth.
Clover and alfalfa show reduction of internode length and swollen stems.
Hosts
More than 400 host plants have been described for D. dipsaci. The species is subdivided in races. The Allium race also attacks oats, sugar beet, Swiss chard, spinach and legumes (fababean, bean, pea, soybean).
Medicago sativa, Glycine max, Trifolium spp.
Geographic distribution
Cosmopolitan, except tropical lowlands; temperate regions where it is one of the most devastating plant parasites; USA.
Biology and transmission
The nematode is carred inside the seed, and it is a seed and soil borne nematode.
Nematode is a migratory endoparasite. At the beginning of the crop season, 4th-stage juvenile enters young tissues, especially seedlings when below the soil surface. Feeding breaks down middle lamellae; nematode probably secretes a pectinase enzyme; plant parts become ‘crisp’ and are easily broken. Migration on plant parts above ground requires free water, and may occur after rain or sprinkler irrigation. Nematode enters through stomata or by direct penetration.
Cardinal temperatures for nematode activity and infection are 10oC - 22oC -30oC. In soil, they survive as fourth stage larvae at temperatures not exceeding 35oC.
Infestation occurs readily in heavier soils and during times of high rainfall or in sprinkler-irrigated areas.
Nematode is spread around field by equipment, irrigation; spreads readily in tail water.
Predisposes alfalfa to Phytophthora megasperma.
Detection/indexing method
- At CIAT: No significant importance
- At ICARDA:, Nematode extraction
References and further references
http://plpnemweb.ucdavis.edu/Nemaplex/Taxadata/G042S1.HTM#Distribution:
Diekmann M. 1997. FAO/IBPGR Technical guidelines for the safe movement of Germplasm. No. 18. Allium spp. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Root-knot nematode infestation
Scientific Name
Meloidogyne spp.
Symptoms
Root knot nematode infestation includes wilting, loss of vigor, yellowing and other symptoms similar to a lack of water or nutrients. Plants often wilt during the hottest part of the day, even with adequate soil moisture, and leaves may turn yellow. Fewer and smaller leaves and fruits are produced, and plants heavily infested early in the season may die.
Root knot nematodes usually cause distinctive swellings, called galls, on the roots of affected plants. Infestations of these nematodes are fairly easy to recognize by digging up a few plants with symptoms, washing or gently tapping the soil from the roots, examining the roots for galls. The nematodes feed and develop within the galls, which may grow to as large as 1 inch in diameter on some plants but are usually much smaller. The water- and nutrient-conducting abilities of the roots are damaged by the formation of the galls. Galls may crack or split open, especially on the roots of vegetable plants, allowing the entry of soil-borne, disease-causing microorganisms.
Root knot nematode galls are true swellings and cannot be rubbed off the roots, as can the beneficial nitrogen-fixing nodules on the roots of legumes.
Hosts
Root knot nematodes have a broad host range of more than 200 reported plants.
As a genus, they are reported as parasites of over 3000 host plants, and individual species often have a wide host range. Some 874 crop species act as hosts of the 7 or 8 species commonly occurring in the western U.S.
Geographic distribution
Southeast Asia, South America, USA
Biology and transmission
The root-knot nematodes (Meloidogyne spp.) form easily recognized galls on the roots. Galls result from growth of plant tissues around juvenile nematodes which feed near the centre of the root. Root-knot gall tissue is firm without a hollow centre, and is an integral part of the root; removing a root-knot gall from a root tears root tissue.
They are difficult to control and can be spread easily from garden to garden in soil (for example, on tools, boots, etc.) and plant parts.
Certain marigolds (Tagetes) suppress root knot nematodes. The effect of marigolds is greatest when they are grown as a solid planting for an entire season. When grown along with annual vegetables or under trees or vines (intercropping), nematode control is usually not very good. As with other cultural control methods, nematode populations will rapidly increase as soon as susceptible crops are grown.
Root knot nematodes may feed on the roots of grasses and certain legumes without causing galling.
Damage is most serious in warm, irrigated, sandy soils.
Detection/indexing method
- At CIAT: Visualization in Stereomicroscopy and Microscopy
Procedure followed at the center in case of positive test
- Species rotation in regeneration fields.
References and further reading
http://plantclinic.cornell.edu/FactSheets/nematodes/nematodes.htm
http://www.ipm.ucdavis.edu/PMG/PESTNOTES/pn7489.html
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
Best practices for the safe transfer of forage legume germplasm
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell); ICARDA, Syria (Siham Asaad).
|
Flowchart 1.Germplasm management of Beans and Tropical Forages (Click to increase the size) |
The agreement between the CIAT and the International Treaty on Plant Genetic Resources for Food and Agriculture implies of the Genetic Resources Program (GRP) the conservation on behalf of the countries of 65,505 materials for 745 plant species of bean, cassava and tropical pastures as the biological patrimony of 141 countries. This responsibility of conservation is associated with the distribution of samples of this patrimony (517,916 samples distributed to 136 countries to the date) according to a regulation defined by the countries inside the Agreement, and phytosanitary procedure established by the Colombian Agricultural Institute.
The job of the GRP is to safeguard the genetic diversity of beans, cassava, forages, and their wild relatives through a mix of conservation methods, both in situ (in a natural outdoor habitat) and ex situ (in the controlled environment of a gene bank). Among the GRP's activities are research to improve conservation methods (including ways to minimize risks to the collections); screening germplasm for diseases and certifying it; duplicating materials of the collection; collecting or otherwise acquiring novel materials; recording passport, characterization and evaluation data for accessions of the collections (See Flowchart 1).
Phytosanitary risks are associated with international movement of germplasm, especially the inadvertent transport of pathogens and pests of quarantine significance. To minimize the phytosanitary risks associated with exchange germplasm and to ensure that it is free from pests and pathogens of quarantine significance; CIAT has implemented regulatory measures and safeguards to complement quarantine guidelines. The process, wich is supervised by the the Agricultural Colombian Institute (ICA), includes the following activities:
- Minimize the risk of accidental introduction of exotic pest and pathogens into Colombia;
- Inspect plant and facilities in screenhouses and grenhouses where the imported germplasm is being incresased;
- Inspect plants in fields and greenhouses where the germplasm intended for international export is produced; and
- Determine the seed health status of germplasm for international export.
The responsibility over the areas dealing with animal and plant health, with regard to international trade, has been bestowed upon the Agricultural Colombian Institute, within regulatory decree 1840 of 1994 “for which (ICA) has the mission of preventing the risk of the entry, spread, and establishment of exotic diseases, those of national sanitary concern, and of chemical risks, and protecting the sanitary quality of animals, plants and products that are exported, to minimize losses in animal-plant production and contribute to the security in foodstuffs”.
ICA has established quarantine procedures to regulate the introduction of plant germplasm and the issuing of phytosanitary certificates that accompany exports. ICA has a Plant Quarantine Station at an altitude of 2600 m (4o 42’ N latitude and 74o 12’W longitude) near Mosquera (Cundinamarca), about 15 km west of Santafé de Bogotá. ICA provides the regulatory mechanism for germplasm exchange following guidelines of the International Plant Protection Convention (IPPC). The recommendations of the IPPC were adopted by Colombian Congress in 1981 and implemented under decrees 501 of 1989.
In order to facilitate the importation and exportation, the ICA has developed the SISPAP, which may accessed through the ICA webpage in which the parties interested in importing and exporting plant products may become aware of the following via internet.
In Colombia, additional guidelines to reduce phytosanitary risks are in place. These safeguards are implemented according to the geographic origin and economic importance of the plant species concerned, and characteristic of potential pathogens and pests. Currently, ICA has a plant quarantine agreement with CIAT establishing which guidelines and safeguards are updated to facilitate germplasm exchange according to national and international requirements. An agreement was signed in 1981 between CIAT and ICA for quarantine procedures to regulate the introductions of germplasm into Colombia. The agreement permits the transit of seed through customs and quarantine stations according to the level of potential risk of introducing pests and diseases no yet reported in Colombia. High risk areas include Africa, Asia, and some European countries. The agreement covers not only crops for wich CIAT has a mandate but also other crops of economic importance to Colombia.
After clearance, materials pass through a step of multiplication in greenhouse stage, and the harvested seeds are subsequently planted in isolated fields. During these two steps, a phytosanitary follow-up is carried out. Plants showing any symptom of fungal, bacterial or viral disease are destroyed.
CIAT facilities for germplasm health testing are designed as a multifunctional laboratory to test seeds and tissues for fungi, bacteria, viruses, and occasionally nematodes and insects (See table below). The purpose of the Germplasm Health Laboratory (GHL) is to ensure that the designated germplasm is kept under the international phytosanitary standards for each crop commodity, and also to ensure that the germplasm distributed by GRP is free of diseases of quarantine importance (listed in the table below).
The ICA Plant Quarantine Officer, stationed at CIAT, carries out field and greenhouses inspections and issues "ICA Phytosanitary Certificate" bases upon those inspections and results obtained by GHL. This document accompanies all out-going germplasm from Colombia (“decrees 1840 of 1994 articule 3”).
The germplasm leaving CIAT and the one used for exchange, conservation, and characterization in the GRP are multiplied in isolated fields under favorable ecological conditions with supervision by ICA quarantine officers. The GRP has seed multiplication sites in Popayan (Cauca) and Santander de Quilichao (Cauca). The harvested seeds are analyzed by GHL prior to shipment abroad or long-term conservation.
Seed health testing activities include:
- Reception, registration, sampling and storage of incoming material.
- Preparation of working samples for testing.
- Analysis.
The seed health testing methods used at CIAT for beans and tropical pastures are summarized in The Handbook of Procedures of the Germplasm Health Laboratory (see also Flows charts 2, 3, 4, 5 below, for a quick reference).
To detect pathogens of quarantine significance, the GHL uses the methodologies recommended by CIAT pathologists and virologists (see table below). When a recipient country has additional requirements, the GHL carries out additional tests wherever possible to comply with the specific quarantine regulations of the recipient country. The GHL realizes additional researches in the management and characterization of pathogens of quarantine importance and in the standardization of new methodologies of diagnosis that are more effective and sensitive.
ILRI carries out virus detection tests before each regeneration. Seeds are withdrawn from the genebank, scarified and germinated using appropriate conditions for the species. Seedlings are transferred to sterilized compost in seedling trays when the radicle is from 1-5mm long and grown in a virus screened area until one to three leaf stage. Visual inspection is carried out and seedlings are batch tested in batches of 10 seedlings using TBIA for common legume viruses. Individual seedling tests are performed on any positive batches for confirmation. Clean seedlings are released to the field for regeneration.
If the seedlings are infected with seed borne pathogens and more original seeds are available for a second regeneration, the seedlings are destroyed by incineration. If no original seeds are available, the seedlings are transferred to large pots and retained in a virus screened area for seed production. Since virus transmission is rarely 100%, the seeds are harvested from infected plants and germinated. The seedlings are tested as above to identify clean seedlings for release to the field. If all seeds are infected thermotherapy and meristem culture are used to eliminate the virus and obtain clean plantlets in in vitro culture. Rooted plantlets can be acclimatized in the greenhouse and these plants used as a source of clean seeds.
Many forage legumes are perennial and can be infected in the field or are direct sown and should be tested during the growing season. Plants in the field are regularly inspected for virus symptoms and samples taken for testing with either TBIA or ELISA for common legume viruses. Plants that test positive for seed borne virus diseases are rogued from the field.
Field inspection is also carried out for pathogenic fungi and insects and any infection is controlled through field management. Regeneration and field genebank plots are sprayed with fungicides or insecticides at early signs of infection.
ILRI uses a range of different methods to detect pathogens of forage legume germplasm. Click the forage legume health table for specific species information about health diagnosis methods to detect some of these diseases. Detailed protocols were developed for the detection of:
- Virus (ELISA and TBIA).
- Fungi (seed washing technique; blotter test; potato dextrose agar; oatmeal agar).
References and further reading
Cuervo MI, Balcazar MS, Ramirez JL, Medina CA, Debouck D. 2009. Manual de operaciones laboratorio sanidad de germoplasma – unidad de recursos genéticos. GRP, CIAT, Colômbia. 72 pp. Click here to open publication
More Articles...
- Viruses - forage legume
- Insects - forage legume
- Weeds (Forage legumes)
- Safe transfer of forage grass germplasm
- Import/export of forage grass germplasm
- Guidelines for safe transfer of forage grass germplasm
- Bacteria - forage grass
- Fungi - forage grass
- Nematodes - forage grass
- Best practices for safe transfer of forage grass germplasm
Subcategories
-
main
- Article Count:
- 11
-
Stog
- Article Count:
- 2
-
Stog-rice
- Article Count:
- 7
-
Stog-sorghum
- Article Count:
- 11
-
Stog-common-bean
- Article Count:
- 10
-
stog-forage-legume
- Article Count:
- 10
-
stog-forage-grass
- Article Count:
- 11
-
stog-maize
- Article Count:
- 9
-
stog-chickpea
- Article Count:
- 10
-
stog-millets
- Article Count:
- 12
-
stog-barley
- Article Count:
- 10
-
stog-groundnut
- Article Count:
- 9
-
stog-pigeon-pea
- Article Count:
- 8
-
stog-wheat
- Article Count:
- 10
-
stog-lentil
- Article Count:
- 9
-
stog-cowpea
- Article Count:
- 10
-
stog-faba-bean
- Article Count:
- 9
-
risk management
- Article Count:
- 4
-
decision support tool
- Article Count:
- 3
-
stog-clonal
- Article Count:
- 23
-
developing strategies
- Article Count:
- 4