CGKB News and events Management strategies
Safe transfer of cowpea germplasm
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
IITA, as one of the 15 CGIAR centers, has the mandate for care and maintenance of cowpea germplasm.
Information is included on:
- Import and export requirements.
- Technical guidelines for the safe movement of germplasm and detection of relevant pathogens and pests.
- Best practices in place at IITA.
Guidelines for safe transfer of cowpea germplasm
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
Technical Guidelines for the safe transfer of germplasm and protection of CGIAR Germplasm Banks: IITA
Seed Crops
Xanthomonas axonopodis pv. Vignae
Best practices for safe transfer of cowpea germplasm
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
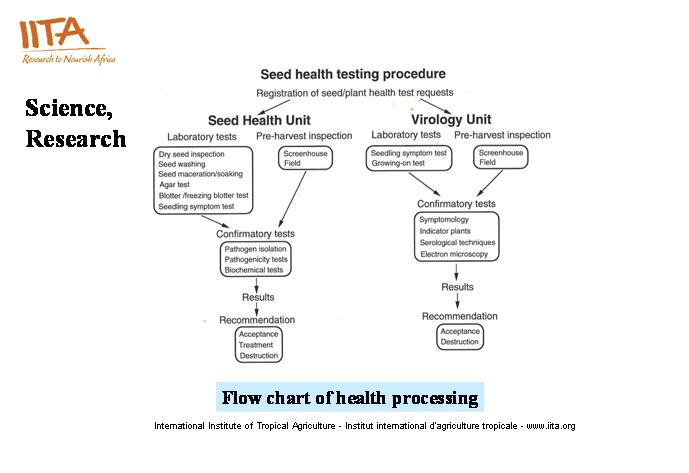
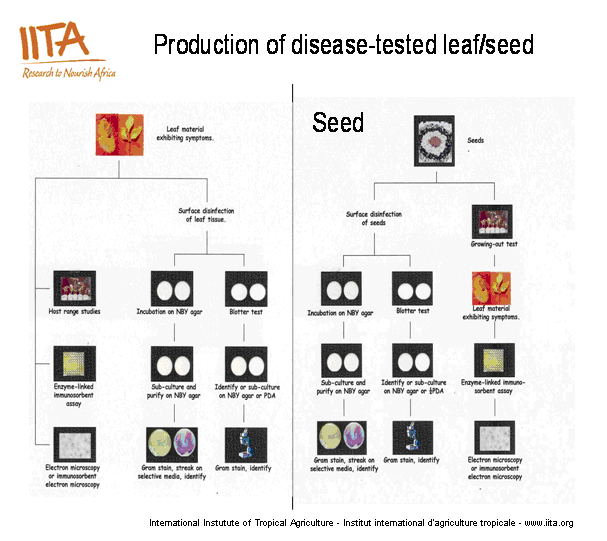
Seed Health Tests and Procedures
Protocols developed for the detection and identification of plant pathogens in the germplasm unit.
The general procedure for testing seeds and plant vegetative parts for the presence of pathogens is presented in this diagram below. Except in some specific cases requiring some special media or modified media for culturing
Viruses - cowpea
Contributors to this section: IITA, Nigeria (M. Ayodele, L. Kumar).
|
Contents: |
Scientific name
Cowpea mosaic virus (CpMV) (genus Comovirus)
Other scientific name
Cowpea mosaic comovirus
Importance
High
Significance
Not reported.
Symptoms
Not reported.
Hosts
Reported hosts are Vigna unguiculata, Glycine max , (soyabean)Vigna umbellata , (Rice- bean)
Geographic distribution
India, Pakistan, Nigeria, Togo
Biology and transmission
Not reported.
Detection/indexing methods used at IITA
- A combination of methods are used for detection and identification
- Methods based on biological properties of the virus
- Growing out test : Symptomatology. Symptoms used to characterized the viruses
- Visual inspection : This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR)
Treatment/control
Strategies for treatment are directed towards prevention of virus infection which are:
- Planting virus free materials
- Controlling vectors where applicable
- No imports of germplasm from countries known to harbour the virus
- Host plant resistance
- Plants with symptoms are rogued during active growth
Procedures in case of positive test
- At IITA, all lines testing positive are discarded after different diagnostic tests have been conducted. There are no economic chemical agents effective against these plant viruses. Hot water is sometimes used, but most of the time when used as seed treatment, the virus is not eliminated from the seeds and the seed quality is reduced.
- If germplasm material is valuable, for import / export,the seeds are grown under containment, inspection during active growth, rogue plants with symptoms and incinerate.
- Serological testing of symptomless lines by ELISA and PCR
- Harvested lines found free from the virus are released to breeders and / or recommended for international distribution.
References and further reading
Naidu RA, d’Hughes J. 2001, Methods for the detection of plant viruses. In Proceedings of the Plant Virology in Sub- Saharan Africa. Conference organized by IITA, 4-6 June 2001.
Scientific name
Blackeye cowpea mosaic virus (BICMV) (genus Potyvirus)
Other scientific name
Bean common mosaic virus strain blackeye cowpea
Importance
High
Significance
Yield reduction from expected 2500kg/ha to 50kg/ha was reported in fields infected with BlCMV in India (Puttaraju et al. (2000)
Cowpea varieties inoculated with BlCMV at the primary leaf stage showed 92-100% infection at first trifoliate leaf.
Iizuka (1990) reported that in field trials, the virus reduced yield of adzuki beans (Vigna angularis) by 33%.
Symptoms
Growth stages and plant parts affected by the virus are seedling, and vegetative stages, the leaves and the whole plant.
Leaves: discoloration, mosaic, mottling, vein banding, vein chlorosis, vein yellowing, leaf deformation and yellow spots.
Seeds : shrivelling
Whole plant: severe green and yellow mosaic, vein banding, mottle, blistering, leaf roll and growth reduction
Presence of virus in the cotyledons, and embryo axes in mature cowpea seeds was reported by Provvidenti, 1986). Sekar and Sulochana (1988)
The spread of BICMV in the fields is effected by aphids after initial seed transmission from infected seeds planted.
Hosts
Reported major hosts areVigna unguiculata ,, Arachis hypogaea ,, Glycine max , Vigna angularis , Vigna mungo , Vigna radiata , , Voandzeia subterranea , (bambara groundnut)(mung bean)(black gram)(adzuki bean)(soyabean)(groundnut)(cowpea) Desmodium incanum, D. tortuosum and Sphenostylis stenocarpa have also been reported as natural hosts. Taiwo et al ( 1982), Brunt et al. (1990), Zhao et al. (1991b), Fery and Dukes (1992).
Geographic distribution
Cosmopolitan.
Biology and transmission
The virus is vector transmitted, mostly by aphids in a non-persistent manner. From experimental results some aphid species have been found to be vectors of the virus such as Aphis craccivora (Bashir and Hampton, 1994, Aphis gossypii ( Mali et al., 1988)
Macrosiphum euphorbiae Murphy et al., 1987), and Myzus persicae (../Jesse Consult Mexico Nov/RefPtr=3N4ceb); Mali et al., 1988].
The virus is seed borne and seed transmitted (Tsuchizaki et al., 1986). Dijkstra et al., 1987
Seedborne infection of the virus has been detected with incidences as high as 50% in cowpea seed by Zettler and Evans, 1972; and Gillaspie et al., 1993.
Detection/indexing methods
- A combination of methods is used for detection and identification
- Methods based on biological properties of the virus
- Growing out test : Symptomathology . Symptoms used to characterized the viruses
- Visual inspection, symptoms This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR).
Treatment/control
Strategies for treatment are directed towards prevention of virus infection which are:
- Planting virus free materials
- Controlling vectors where applicable
- No imports of germplasm from countries known to harbour the virus/ additional declaration that seeds for international distribution were grown in locations known to be free of the virus
- Imports subjected to post entry processing on arrival
- Host plant resistance
- Plants with symptoms are rogued during active growth
Procedures in case of positive test at IITA
- All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted. Hot water has been recommended, but most of the time when used for seed treatment, the seeds deteriorate and the viuruses not eliminated
- If germplasm material is valuable, grow import / export seeds under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders and / or recommend for international distribution
References and further reading
Bashir M, Hampton RO. 1994. Seed and aphid transmission of some isolates of blackeye cowpea and cowpea aphid-borne mosaic potyviruses. Pakistan Journal of Phytopathology, 6(2):140-146.
Brunt A, Crabtree K, Gibbs A. 1990. Viruses of Tropical Plants. Wallingford, UK: CAB International
Dijkstra J, Bos L, Bouwmeester HJ, Hadiastono T, Lohuis H. 1987. Identification of blackeye cowpea mosaic virus from germplasm of yard-long bean and from soybean, and the relationships between blackeye cowpea mosaic virus and cowpea aphid-borne mosaic virus. Netherlands Journal of Plant Pathology, 93(3):115-133.
Fery RL, Dukes PD. 1992. 'Carolina Crowder' southernpea. HortScience, 27(12):1335-1337.
Gillaspie AGJr, Hopkins MS, Pinnow DL. 1993. Relationship of cowpea seed-part infection and seed transmission of blackeye cowpea mosaic potyvirus in cowpea. Plant Disease, 77(9):875-877
Iizuka N. 1990. Studies on virus diseases of adzuki bean (Vigna angularis Wight) in Japan. Bulletin of the Tohoku National Agricultural Experiment Station, No. 82:77-113
Mali VR, Mundhe GE, Patil NS, Kulthe KS. 1988. Detection and identification of blackeye cowpea mosaic and cowpea aphid borne mosaic viruses in India. International Journal of Tropical Plant Diseases, 6(2):159-173.
Murphy JF, Barnett OW, Witcher W. 1987. Characterization of a blackeye cowpea mosaic virus strain from South Carolina. Plant Disease, 71(3):243-248.
Provvidenti R. 1986. Seed transmission of blackeye cowpea mosaic virus in Vigna mungo. Plant Disease, 70(10):981
Puttaraju HR, Prakash HS, Shetty HS. 2000. Field incidence, seed-transmission and susceptibility of cowpea varieties with reference to Blackeye Cowpea Mosaic Potyvirus. Seed Research, 28(2):196-202
Sekar R, Sulochana CB. 1988. Seed transmission of blackeye cowpea mosaic virus in two cowpea varieties. Current Science, 57(1):37-38
Taiwo MA, Gonsalves D, Provvidenti R, Thurston HD. 1982. Partial characterization and grouping of isolates of blackeye cowpea mosaic and cowpea aphidborne mosaic viruses. Phytopathology, 72(6):590-596.
Tsuchizaki T, Senboku T, Iwaki M, Kiratiya-Angul S, Srithongchai W, Deema N, Ong CA. 1986. Blackeye cowpea mosaic virus from asparagus bean (Vigna sesquipedalis) in Thailand and Malaysia. Technical Bulletin of the Tropical Agriculture Research Center, No.21:213-218.
Zettler FW, Evans IR. 1972. Blackeye cowpea mosaic virus in Florida: host range and incidence in certified cowpea seed. Proceedings of the Florida State Horticultural Society, 85:99-101.
Scientific name
Cowpea severe mosaic virus (CpSMV) (genus Comovirus)
Other scientific names
Cowpea severe mosaic comovirus, Puerto Rico cowpea mosaic virus
Importance
High
Significance
Yield losses of 60-80% caused by CSMV were reported in Brazil
Symptoms
Not reported.
 Cowpea severe mosaic virus (photo: IITA) |
Hosts
The major hosts of this virus are Vigna unguiculata (cowpea) Canavalia ensiformis (gotani bean), Crotalaria juncea (sunn hemp), Glycine max (soyabean), Phaseolus vulgaris (common bean), Psophocarpus tetragonolobus (winged bean), Vigna radiata (mung bean).
Geographic distribution
Widespread in North , South and Central America, Brazil, Mexico, Peru, Cuba, Trinidad and Tobago, Pakistan, Senegal, Nigeria.
Biology and transmission
Seed borne, sap and insect transmitted mostly beetles such as Cerotoma sp.
Detection/indexing methods at IITA
- A combination of methods is used for detection and identification
- Methods based on biological properties of the virus
- Growing out test : Symptomathology . Symptoms used to characterized the viruses
- Visual inspection :This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR).
Treatment/control
- Planting virus free materials
- Controlling vectors where applicable
- No imports of germplasm from countries known to harbour the virus/ additional declaration that seeds for international distribution were grown in locations known to be free of the virus
- Imports subjected to post entry processing on arrival
- Host plant resistance
- Field inspection
- Rogue plants with symptoms and discard
Procedures in case of positive test at IITA
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted
For import
- If germplasm material is valuable, grow import material under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research.
For export
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally
References and further reading
Alconero R, Santiago. (1973). Phytopathology 63, 120-123
Diaz A. (1974). Phytopathology 64, 767.
Lima JAA, Nelson MR. (1977). Pl. Dis. Reptr. 61, 864-867
Shepherd RJ. (1964). Phytopathology 54, 466-473
Scientific name
Cowpea aphid-borne mosaic virus (CAMV, CABMV) (genus Potyvirus)
Other scientific names
Cowpea Moroccan aphid-borne mosaic virus (Fischer & Lockhart, 1976)
Importance
High
Significance
Complete loss of a cowpea crop in northern Nigeria resulting from CABMV attack under irrigated field conditions was reported by Raheja and Leleji (1974). A yield loss of 13-87% due to natural infection of cowpea by CABMV was reported in Iran by Kaiser and Mossahebi, ( 1975). While Kannaiyan and Haciwa, (1993) reported a loss 48-60% in Zambia).
Symptoms
All the plant stages and parts are affected: flowering , fruiting, seedling and vegetative stages in addition to the pods, growing points, inflorescence, leaves, seeds, stems and whole plant.Symptoms vary according to the cowpea cultivar and the existing CABMV race. Shoyinka et al., (1997) reported that CABMV symptoms observed on cowpea under field conditions were extremely variable.
Symptoms expressed on the different parts are:
-
Leaves: primary leaves show vein-clearing, vein-yellowing, diffused chlorotic spots / patches, or an intense chlorosis (Phatak, 1974; Bashir, 1992).
-
On trifoliate leaves: vein-yellowing, yellow mosaic with or without dark-green, irregular vein-banding and blistering, deformation, puckering and stunting (Kaiser and Mossahebi, 1975), mosaic pattern, cupping ,distortion and necrotic lesions. Williams (1975)
-
Inflorescence: lesions, virus in the pollens, anthers and ovaries, the plumule, and cotyledons, Phatak (1974), Tsuchizaki et al. (1970)
-
Growing points: lesions; abnormal forms
-
Stem: necrosis , abnormal forms and growth.
-
Pods: deformation , lesions; discoloration
-
Seeds: reduction in seed size, discoloration, lesion and loss of viability (Kaiser and Mossahebi, 1975).
-
Whole plant: Systemic mosaic, stunting, distortion; rosetting
|
Cowpea aphid-borne mosaic virus (photo: IITA) |
Hosts
Although cowpea (Vigna unguiculata ) is the main host of this virus other major hosts include, Sesamum indicum (sesame ),Voandzeia subterranea . While (bambara groundnut)Glycine max , (soyabean)Pachyrhizus erosus , (yam bean)Pisum sativum , (pea)Vigna radiata are reported as minor hosts.(mung bean)
Glycine max, Cajanus cajan, Cicer arietinum, Lablab purpureus, Vigna subterranea and Lens culinaris were reported as symptomless carriers by Mazyad et al.,( 1981)
Edwardson and Christie (1986) reported that CABMV infected 53 species in 28 genera of the Leguminosae. The virus is also said to infect 13 other families among which are Amaranthaceae, Aizcaceae, Chenopodiaceae, Cucurbitaceae, Hydrophyllaceae, Labiatae, Iridaceae, Leguminosae, Scrophulariaceae, Polygonaceae, Solanaceae and Pedaliaceae. Isolates from the different parts of the world have different hosts range (Bock, 1973).
Geographic distribution
The virus is worlwide in distribution. It is considered to be a major and widespread disease of cowpea through out sub-Saharan Africa (Bock, 1973; Ladipo, 1976; Thottappilly and Rossel, 1985; Burke et al., 1986
The virus has also been reported in Europe, Asia, Africa, Brazil, USA, Australia and Papua New Guinea
Biology and transmission
CABMV is seed borne and seed transmitted, (Allen, 1983; Rossel and Thottappilly, 1990). The virus survives in infected seed, volunteer host plants and in viruliferous aphids. Thottappilly, (1992)
The virus is transmitted mechanically ( sap), and vector transmitted by several aphid species in a stylet-borne non-persistent manner, and Aphis craccivora is the most efficient vector (Bock, 1973;Atiri et al., 1984, 1986). The aphid species reported to be vectors of CABMV in addition to Aphis craccivora, are A. gossypii, A. spiraecola, A. medicaginis, A. fabae, A. citricola, A. sesbaniae, Macrosiphum euphorbiae, Myzus persicae, Rhopalosiphum maidis, Cerataphis palmae and Acyrthosiphon Dijkstra et al., 1987; Mali et al., 1988; Thottapilly, 1992; Thottapilly and Rossel, 1992; Roberts et al., 1993; Bashir and Hampton, 1994).
Detection/indexing methods at IITA
- Growing out test: in screen houses/ containment facility to determine presence/ absence of virus symptoms in the seedlings growing from the virus-infected seeds.
- Infectivity test: presence of virus assayed by inoculating extracts of seed or seedlings on to indicator hosts under containment facility
- Serological tests: most reliable and effective methods for the detection of seedborne viruses and virus from plant tissues
- ELISA: Enzyme-linked immunosorbent assay. PCR
Treatment/control
- No seed treatment has yet been reported to eliminate CABMV directly from seed
- IITA has resistant cowpea lines
- Grow resistant cowpea cultivars against aphids attack and CABMV infection
- Production of virus-free seed to supply to breeders.
- Production of seeds for international distribution under certification scheme
- Field inspection during active growth and roguing of diseased plants
Procedures in case of positive test at IITA
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted.
For Import:
- If germplasm material is valuable, grow import material under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research
For export:
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally
References and further reading
Allen DJ. 1983. Disease resistance in crop improvement. In: The Pathology of Tropical Food Legumes. Chichester, UK: John Wiley and Sons, 210-213.
Atiri GI, Ekpo EJA, Thottappilly G. 1984. The effect of aphid-resistance in cowpea on infestation and development of Aphis craccivora and the transmission of cowpea aphid-borne mosaic virus. Annals of Applied Biology, 104(2):339-346.
Atiri GI, Enobackhare DA, Thottappilly G. 1986. The importance of colonizing and non-colonizing aphid vectors in the spread of cowpea aphid-borne mosaic virus in cowpea. Crop Protection, 5(6):406-410
Bashir M. 1992. Serological and biological characterization of seed-borne isolates of blackeye cowpea mosaic and cowpea aphid-borne mosaic potyviruses in Vigna unguiculata (L.) Walp. PhD Thesis, Oregon State University, Corvallis, Oregon, USA.
Bashir M, Hampton RO. 1994. Seed and aphid transmission of some isolates of blackeye cowpea and cowpea aphid-borne mosaic potyviruses. Pakistan Journal of Phytopathology, 6(2):140-146
Bock KR. 1973. East African strains of cowpea aphid-borne mosaic virus. Annals of Applied Biology, 74(1):75-83;
Burke DW, Ditshipi P, DeMooy CJ. 1986. Virus diseases of cowpeas in dryland and irrigated plots in Botswana. Plant Disease, 70(8):801
Dijkstra J, Bos L, Bouwmeester HJ, Hadiastono T, Lohuis H. 1987. Identification of blackeye cowpea mosaic virus from germplasm of yard-long bean and from soybean, and the relationships between blackeye cowpea mosaic virus and cowpea aphid-borne mosaic virus. Netherlands Journal of Plant Pathology, 93(3):115-133
Edwardson JR, Christie RG. 1986. Viruses infecting forage legumes. Vol. II. Monograph No. 14. Agriculture Experimental Station, University of Florida, Gainesville, Florida, USA
Kaiser WJ, Mossahebi GH. 1975. Studies with cowpea aphid-borne mosaic virus and its effect on cowpea in Iran. Plant Protection Bulletin, FAO, 23(2):33-39
Kannaiyan J. Haciwa HC. 1993. Diseases of food legume crops for the scope of their management in Zambia. FAO Plant Protection Bulletin, 41:73-90.
Ladipo JL. 1976. A vein-banding strain of cowpea aphid-borne mosaic virus in Nigeria. Nigerian Journal of Science, 10:77-86.
Mali VR, Mundhe GE, Patil NS, Kulthe KS. 1988. Detection and identification of blackeye cowpea mosaic and cowpea aphid borne mosaic viruses in India. International Journal of Tropical Plant Diseases, 6(2):159-173
Mazyad HM, El-Hammady M, El-Amrety AA, El-Din ASG. 1981. Studies in cowpea aphid-borne mosaic virus in Egypt. Agricultural Research Review, 59(2):167-178
Phatak HC. 1974. Seed-borne plant viruses - Identification and diagnosis in seed health testing. Seed Science and Technology, 2:3-155.
Raheja AK, Leleji OI. 1974. An aphid-borne virus disease of irrigated cowpea in Northern Nigeria. Plant Disease Reporter, 58(12):1080-1084
Roberts JMF, Thottappilly G, Hodgson CJ. 1993. The ability of Aphis craccivora, A. gossypii and A. citricola to transmit single and mixed viruses to cowpeas. Journal of Phytopathology, 138(2):164-170
Rossel HW, Thottappilly G. 1990. Possible dependence of geographical distribution of virus diseases of cowpea in African agroecological parameters. In: Allen DJ, ed. Proceedings of Working Group Meeting on Virus Diseases of Beans and Cowpeas in Africa. CIAT Africa Workshop Series No. 13. Cali, Colombia: Centro Internacional de Agricultura Tropical, 33-37.
Shoyinka SA, Thottappilly G, Adebayo GG, Anno-Nyako FO. 1997. Survey on cowpea virus incidence and distribution in Nigeria. International Journal of Pest Management, 43(2):127-132
Thottappilly G, Rossel HW. 1985. Worldwide occurrence and distribution of virus diseases, In: Singh SR., Rachie RO, eds. Cowpea Research, Production and Utilization. Chichester, UK: John Wiley and Sons, 155-171.
Thottappilly G, Rossel HW. 1992. Virus diseases of cowpea in tropical Africa. Tropical Pest Management, 38(4):337-348
Tsuchizaki T, Yora K, Asuyama H. 1970. The viruses causing mosaic of cowpea and azuki bean, and their transmissibility through seeds. Annals of the Phytopathological Society of Japan, 36:112-120.
Scientific name
Cowpea mottle virus (CPMoV) (genus Carmovirus)
Importance
High
Significance
The impact of infection on the yield of cowpea is not known but yield losses of 64-80% in groundnuts were reported in Kenya by Bock et al., 1976, 1977). Other unquantified yield losses, of groundnuts, soyabeans, bambara groundnuts (Vigna subterranea) and winged beans (Psophocarpus tetragonolobus ) have been reported by Fauquet et al., (1979); Fortuner et al., (1979); Dubern and Dollet, (1981); Thouvenel et al., (1982); Fauquet and Thouvenel, (1987); Saleh et al., (1989); and Reddy, (1991).
Symptoms
The virus affects all growing stages of the plant, flowering, podding, and seedling stages. The leaves and the whole plant are also infected.
Symptoms exhibited on cowpea are :
Leaves: mild to severe chlorotic mottling, distortion, stunting.
Whole plants: stunting.
|
|
|
|
|
Cowpea mottle virus (photos: IITA) |
||
Hosts
Although the natural hosts of CPMV are leguminous species, the virus also occurs naturally in tomatoes in Israel and Nigeria.
The major hosts are: Vigna unguiculata (cowpea), Arachis hypogaea (groundnut), Glycine max (soyabean), Lycopersicon esculentum (tomato), Phaseolus vulgaris (common bean).
The minor recorded hosts are Calopogonium mucunoides (calopo (Australia)), Mucuna pruriens (Buffalobean), Phaseolus lunatus (lima bean), Phaseolus radiata, Psophocarpus tetragonolobus (winged bean), Vicia faba (broad bean), Voandzeia subterranea (bambara groundnut) while Centrosema pubescens (Centro), Desmodium tortuosum (Florida beggarweed), Stylosanthes gracile , Tephrosia villosa are reported as wild hosts
Geographic distribution
The virus is widely distributed in Africa, Asia, Oceania and South America.
Biology and transmission
Transmitted by whiteflies Bemisia tabaci,(Brunt, 1995), Thottappilly and Rossel, 1992). Seed borne and seed transmitted, (Brunt and Kenten, 1973), (Nain et al., 1994).
Detection/indexing methods at IITA
A combination of methods is used for detection and identification. The methods are based on biological properties of the virus.
- Growing out test : Symptomathology . Symptoms used to characterize the viruses
- Visual inspection: this requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR)
Treatment/control
- No seed treatment has yet been reported to eliminate CABMV directly from seed
- IITA has resistant cowpea lines
- Grow resistant cowpea cultivars against aphids attack and CABMV infection
- Production of virus-free seed to supply to breeders.
- Production of seeds for international distribution under certification scheme
- Field inspection during active growth and roguing of diseased plants
Procedures in case of positive test at IITA
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted.
For Import :
- If germplasm material is valuable, grow import material under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research.
For export:
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally
References and further reading
Bock KR, Guthrie EJ, Meredith G, Njuguna JGM. 1976. Plant pathology: Groundnut viruses. Report of the East African Agriculture and Forestry Research Organisation for 1974. Nairobi, Kenya: East African Agriculture and Forestry Research Organisation, 120-128.
Bock KR, Guthrie EJ, Meredith G, Njuguna JGM. 1977. Plant pathology. Report of the East African Agriculture and Forestry Research Organisation for 1975. Nairobi, Kenya: East African Agriculture and Forestry Research Organisation, 117-124.
Brunt AA. 1995. Genus Carlavirus. In: Murphy F, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, Martelli GP, Mayo MA, Summers MD, editors. Virus Taxonomy: Classification and Nomenclature of Viruses. Sixth Report of the International Committee on Taxonomy of Viruses. Archives of Virology, Supplement 10. Vienna: Springer-Verlag, 475-478.
Brunt AA, Kenten RH. 1973. Cowpea mild mottle, a newly recognized virus infecting cowpea (Vigna unguiculata) in Ghana. Annals of Applied Biology, 74(1):67-74;
Dubern J, Dollet M. 1981. Groundnut crinkle virus, a new member of the carlavirus group. Phytopathologische Zeitschrift, 101(4):337-347;
Fauquet C, Lamy D, Thouvenel J-C. 1979. Viral diseases of winged bean in the Ivory Coast. FAO Plant Protection Bulletin, 27:81-87.
Fauquet C, Thouvenel J-C. 1987. Plant viruses in the Ivory Coast. Initiations, Documentations, Techniques, No. 46. Paris, France:ORSTOM, 243
Fortuner R, Fauquet C, Lourd M. 1979. Diseases of the winged bean in Ivory Coast. Plant Disease Reporter, 63(3):194-199
Nain PS, Rishi N, Bishnoi SS. 1994. Profile of viral diseases of cowpea (Vigna unguiculata) in northern India. Indian Journal of Virology, 10(2):128-136.
Reddy DVR. 1991. Crop profile. Groundnut viruses and virus diseases: distribution, identification and control. Review of Plant Pathology, 70(9):665-678.
Saleh N, Baliadi Y, Horn NM. 1989. Cowpea mild mottle virus naturally infecting groundnut in Indonesia. Penelitian Palawija, 4:32-35.
Thottappilly G, Rossel HW. 1992. Virus diseases of cowpea in tropical Africa. Tropical Pest Management, 38(4):337-348.
Scientific name
Cowpea golden mosaic virus (CGMV) (genus Bigeminivirus)
Importance
High
Significance
Unquantified severe losses reported in Nigeria.
Symptoms
Symptoms are exhibited on the leaves and on the whole plant.
Leaves: yellowing, chlorosis, distortion, blistering, blotches
Whole plant: stunting.
|
Cowpea golden mosaic virus (photos: IITA) |
Hosts
Cowpea.
Geographic distribution
Nigeria, Kenya, Tanzania, Pakistan
Biology and transmission
The virus is transmitted by whiteflies Bemisia specie
Detection/indexing methods used at IITA
A combination of methods is used for detection and identification. The methods are based on biological properties of the virus.
- Growing out test : Symptomatology . Symptoms used to characterized the viruses
- Visual inspection: take into consideration may exhibit similar symptoms This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests : using indicator plants: mechanical sap, grafting, vector
- Physical: Electron microscopy, Serology(ELISA and PCR)
Treatment/control
- Host resistance
- Control of white flies using pesticides
- Production in Pest Free Areas
- Production of virus-free seed to supply to breeders.
- Production of seeds for international distribution under certification scheme
- Field inspection during active growth and roguing of diseased plants
Control of whiteflies (Bemisia specie ) using any of the underlisted pesticides as sprays on the field during active growth:
Chemical control
- Act force 100ml to 20lts water or
- Cyper force 100ml to 20lts water or
- Cyper Diforce 100ml to 20lts water
Protocol
- Spray cowpea with any of the above mentionned insecticide at 7-10 days interval beginning from flower bud initiation.
- In case of severe infestation by whiteflies (Bemisia specie) during seedling stage,
- one spray may be needed before flowering. Normally four applications of insecticide are adequate to control the pests.
- Seed treatment using any available pesticide / seed fumigation using phostoxin
Procedures in case of positive test
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted.
For import:
- For valuable germplasm material , grow imported material under containment, inspection during active growth, rogue plants with symptoms
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research
For export:
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally
References and further reading
IITA. 1977. Highlights of 1976 Research, International Research Institute of Tropical Agriculture, Ibadan, Nigeria, 57 pp
Mushtaq Ahmad. 1978. Pl. Dis. Reptr. 62, 224-226
Scientific name
Cowpea yellow mosaic virus (CYMV)
Other scientific name
Cowpea mosaic comovirus
Importance
High
Significance
Yield losses of 80-100% were reported by Singh and Allen
Symptoms
Symptoms differ with different cultivars
Leaf: mosaic, distortion, blistering
Whole plant: systemic infection, green mottling, stunting, dead
|
Cowpea yellow mosaic virus (photo: IITA) |
Hosts
The hosts include: Vigna unguiculata (cowpea), Chenopodium quinoa (quinoa), Crotalaria juncea (sunn hemp), Glycine max (soyabean), Vigna umbellata (Rice- bean).
Geographic distribution
India, Pakistan, Africa ( Kenya, Tanzania, Nigeria, Togo), Surinam
Biology and transmission
Virus is seed borne and seed transmitted.
Sap transmitted
Vector transmitted by beetles Ootheca mutabilis including other species, grasshoppers, and thrips have been reported as vectors of the virus
Detection/indexing methods at IITA
A combination of methods is used for detection and identification. The methods are based on biological properties of the virus.
- Growing out test : Symptomatology . Symptoms used to characterized the viruses
- Visual inspection: take into consideration may exhibit similar symptoms This requires expertise and adequate field experience and confirmation using confirmatory tests
- Transmission tests: using indicator plants: mechanical sap, grafting, vector
-
Physical: Electron microscopy, Serology(ELISA and PCR)
Treatment/control
- Host resistance
- Production in Pest Free Areas
- Production of virus-free seed to supply to breeders.
- Production of seeds for international distribution under certification scheme
- Field inspection during active growth and roguing of diseased plants.
Control of beetles Ootheca mutabilis and the other vectors using any of the underlisted pesticides as sprays on the field during active growth:
Chemical control
- Act force 100ml to 20lts water or
- Cyper force 100ml to 20lts water or
- Cyper Diforce 100ml to 20lts water
Protocol
- Spray cowpea with any of the above mentionned insecticide at 7-10 days interval beginning from flower bud initiation.
- In case of severe infestation by beetles (Ootheca mutabilis) during seedling stage,
- one spray may be needed before flowering. Normally four applications of insecticide are adequate to control the pests.
- Seed treatment using any available pesticide / seed fumigation using phostoxin
Procedures in case of positive test
All lines testing positive are discarded after different confirmatory diagnostic tests have been conducted
For Import:
- For valuable germplasm material , grow imported material under containment, inspection during active growth, rogue plants with symptoms;
- Serological testing of symptomless lines
- Harvest lines found free from the virus and release to breeders for research
For export:
- Seeds from symptomless lines harvested from the multiplication plots meant for international distribution are grown in the screen houses.
- The lines are inspected during active growth in collaboration with Plant Quarantine officials. Plants with symptoms are rougued and incinerated. Leaf samples and seeds from symptomless plants are tested serologically by ELISA or PCR
- Only disease free lines are distributed internationally.
References
Bock KR. 1971. E. Afri. Agric. For. J. 37, 60-62
Witney WK, Gilmer RM. 1974. Ann. Appl. Biol.77, 17-21
Williams RJ. 1977. Trop.Agric. (Trin.). 54, 61-68
More Articles...
Subcategories
-
main
- Article Count:
- 11
-
Stog
- Article Count:
- 2
-
Stog-rice
- Article Count:
- 7
-
Stog-sorghum
- Article Count:
- 11
-
Stog-common-bean
- Article Count:
- 10
-
stog-forage-legume
- Article Count:
- 10
-
stog-forage-grass
- Article Count:
- 11
-
stog-maize
- Article Count:
- 9
-
stog-chickpea
- Article Count:
- 10
-
stog-millets
- Article Count:
- 12
-
stog-barley
- Article Count:
- 10
-
stog-groundnut
- Article Count:
- 9
-
stog-pigeon-pea
- Article Count:
- 8
-
stog-wheat
- Article Count:
- 10
-
stog-lentil
- Article Count:
- 9
-
stog-cowpea
- Article Count:
- 10
-
stog-faba-bean
- Article Count:
- 9
-
risk management
- Article Count:
- 4
-
decision support tool
- Article Count:
- 3
-
stog-clonal
- Article Count:
- 23
-
developing strategies
- Article Count:
- 4