CGKB News and events Management strategies
Guidelines for the safe transfer of lentil germplasm
Contributors to this page: ICARDA, Aleppo, Syria (Siham Asaad, Abdulrahman Moukahal).
Technical Guidelines for the Safe Transfer of Germplasm and the Protection of CGIAR Germplasm Banks
Pathogens of quarantine significance of lentil (ICARDA)
|
Virus
|
|
Bean yellow mosaic virus (BYMV)
|
|
Broad bean stain virus (BBSV)
|
|
Pea Seed-borne Mosaic Virus (PSbMV)
|
|
Broad bean Mottle Virus (BBMV)
|
|
Fungi
|
|
Didymella lentis (anamorph Ascochyta lentis)
|
|
Botrytis cinerea
|
|
Phoma medicaginis var. medicaginis
|
|
Glomerella lindemuthiana (anamorph Colletotrichum lindemuthianum); C. truncatum
|
|
Fusarium oxysporum f.sp. lentis
|
|
Insects
|
|
Bruchus lentis; Bruchus ervi
|
|
Callosobruchus maculatus; C. chinensis
|
|
Nematodes
|
|
Ditylenchus dipsaci
|
|
Weeds
|
|
Orobanche foetida; Orobanche crenata;
Orobanche ramosa; Orobanche aegyptiaca;
|
|
Cuscuta campestris; Cuscuta hyalina
|
Best practices for the safe transfer of lentil germplasm
Contributors to this page: ICARDA, Aleppo, Syria (Siham Asaad, Abdulrahman Moukahal).
In preparation
Viruses - lentil
Contributors to this page: ICARDA, Aleppo, Syria (Siham Asaad, Abdulrahman Moukahal).
|
Contents: |
Scientific name
Bean yellow mosaic virus (BYMV)
Other scientific names
Bean virus 2 (BV-2), canna mosaic virus, gladiolus mosaic virus, gloriosa stripe mosaic virus
Significance
During our pulse surveys from 2000-2005, BYMV infections were uncommon and found in less than 1% of surveyed crops. However, BYMV was sometimes found in faba bean crops and occasionally in peas with within crop virus incidences of 1-15% and up to 7% respectively. In NSW, a small survey showed that faba bean crops had an average within crop incidence of BYMV of 26% of plants (ranging from 1-63%) (van Leur et al. 2002). Field surveys in 1998-1999 showed that some faba bean and field pea crops were infected with BYMV and the within crop virus incidences were 1-31% and 1-11% respectively. Plot trials showed that lupins infected with the necrotic strain of BYMV can have grain yields reduced by 95%.
Symptoms
Leaves of infected plants show mild mosaic followed by narrowing. New growth from leaf axils shows leaves that are narrow, elongated and light green. Early infections adversely affect plant growth and yield; there may be reduction in leaf size and stunting. Infected plants produce very little seed.
 Bean yellow mosaic (photo: ICARDA) |
Hosts
Wide host range. BYMV is reported to infect nearly 200 species in 14 families.
Legume hosts include lentils, chickpeas, beans, faba beans, green beans, peas, soybeans, peanuts, lupins, lathyrus, lucerne, vetch, clover, and various pasture hosts. The virus also infects horticultural and ornamental plants, such as gladiolus species.
Geographic distribution
Cosmopolitan
Biology and transmission
BYMV is transmitted by more than 50 aphid species in a non-persistent manner: the main species are Acyrthosiphon pisum, Aphis fabae, A. gossypii, Aulacorthum solani, Brevicoryne brassicae, Myzus persicae and Rhopalosiphum maidis. During our surveys in Victoria we have found the following BYMV vectors in pulse crops: Acyrthosiphon kondoi, Aphis craccivora, Aulacorthum solani, Brevicoryne brassicae and Myzus persicae. In WA Acyrthosiphon kondoi, Aphis craccivora, Myzus persicae, Brachycaudus rumexicolens, Lipaphis erysimi, Rhopalosiphum maidis, R. padi, Sitodion miscanthi and Therioaphis trifolii have been reported as BYMV vectors.
The virus is also transmitted through seed of most temperate pulses, including faba beans, field peas, lentils, and lupins and through seed of a number of forage legumes and clovers. In Victoria, we found 18% of lentil seed lots tested had BYMV infections of 0.1-0.9%. In WA, the following BYMV seed transmission is reported: yellow and white lupins 3-6%, field peas 0.3-0.8%, faba beans 0.4%, lathyrus 0.1-0.2% and vetch 0.5%.
Seed transmission of BYMV in medics and clovers has also been reported in WA as follows: Melilotus indica (0.5%), Medicago polymorpha (0.9%), M. truncatula (0.3%), M. indica (1%), Trifolium arvense (0.1%), T. campestre (0.2%) and T. glomeratum (0.05%).
Detection/indexing methods used at ICARDA
- Tissue blot immunoassay (TBIA)
- Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be one of the main sources of BYMV, therefore sowing virus tested seed is recommended and commercial seed tests are available. BYMV causes heavy losses in narrow leafed lupins, so only virus tested seed is recommended for sowing. Virus resistant lupin varieties are now available. BYMV-infected pastures are another major source of the virus, which is then spread to crops by aphids. Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses such as BYMV. Pulse crops should be sown away from legume pastures to minimise the spread of BYMV. The spread of virus can also be reduced by controlling weed hosts from in and around paddocks.
Procedures followed in case of positive test at ICARDA
- The seed lot is destroyed.
References and further reading
Aftab M, Freeman A. 2006. Temperate pulse viruses: Bean yellow mosaic virus (BYMV). Agriculture note AG1266. Melbourne: Department of Primary Industries, Victoria, Australia.
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Bean yellow mosaic. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lentil.htm#Lent7.html. Date accessed 13 April 2010
 |
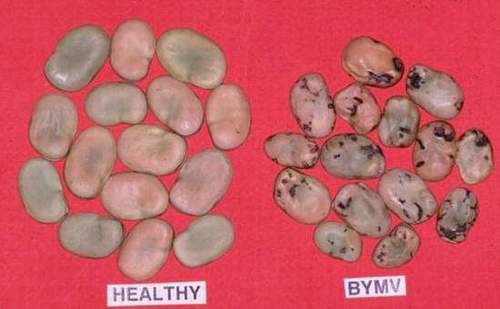 |
|
|
Bean yellow mosaic virus (BYMV) (photos: Department of Primary Industries, Victoria, Australia) |
||
Scientific name
Broad bean stain virus (BBSV)
Other scientific names
Broad bean F1 virus, broad bean Evesham stain virus
Significance
In a study of 19 lentil genotypes in Syria, yield loss due to infection varied between 14% and 61% (Makkouk and Kumari 1990).
Symptoms
The disease is characterized by a mild mottling on the leaves, although this is not easy to recognize due to the small size of lentil leaf.
Affected plants are reduced in growth, which is easily recognized, especially in comparison with healthy plants. Yield of affected plants is also reduced.
Seed from infected plants occasionally show dark staining.
 Healthy lentil seed (right) and seed with BBSV symptoms (left) which include dark staining and distortion.(photo: PaDIL, ICARDA). |
Hosts
The virus is restricted to legumes. In addition to lentil, it also naturally affects faba bean (or broad bean), dry pea and vetch.
Geographic distribution
Europe, Middle East, North Africa, West Asia
Biology and transmission
BBSV is transmitted by insect vectors, the weevilsApion vorax and Sitona spp. It is also transmitted through sap and seed. In 19 lentil genotypes studied in Syria seed transmission rates varied between 0.2% and 32.4% (Makkouk and Kumari 1990).
Detection/indexing methods used at ICARDA
- Tissue blot immunoassay (TBIA)
- Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- It is important to avoid the use of infected seed. Lentil should be planted away from other legume hosts and infected plants should be promptly removed to prevent spread of the disease.
- As BBSV is transmitted by weevils, seed may be treated with Promet (12 ml/kg seed) to help control the vector and hence disease spread.
Procedures followed in case of positive test at ICARDA
- The seed lot is destroyed.
References and further reading
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Broad bean stain. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lent7.Html#_Broad. Date accessed 13 April 2010
Gibbs AJ, Smith HG. 1970. Broad bean stain virus. Descriptions of plant viruses 29. Association of Applied Biologists. [online] Available from URL: http://www.dpvweb.net/dpv/showdpv.php?dpvno=029. Date accessed 13 April 2010
Makkouk KM, Kumari SG. 1990. Variability among 19 lentil genotypes in seed transmission rates and yield loss induced by broad bean stain virus infection. LENS Newsletter 17(2): 31-33.
Plants and Diseases Image Library (PaDIL). Plant biosecurity toolbox: Broad bean stain virus (BBSV). [online] Available from URL: http://www.padil.gov.au/pbt/index.php?q=node/20&pbtID=154. Date accessed 13 April 2010
Scientific Name
Pea Seed-borne Mosaic Virus (PSbMV)
Other scientific names
Pea fizzle top virus, pea leaf rolling virus, pea leaf roll mosaic virus, pea leaf rolling mosaic virus.
Significance
PSBMV is of economic importance in pea, faba bean and lathyrus, mainly due to its effect on seed quality. It has been mistakenly considered to be a minor disease because it often causes only minor yield loss and mild symptoms, However, at the International Centre for Agriculture in the Dry Areas (ICARDA), Syria, glasshouse studies on yield losses due to PSBMV in chickpea, faba bean, lentil and pea showed losses of 66%, 40.5%, 44.6% and 49.2% respectively. In SA, glasshouse experiments showed that pathotypes P1 and P4 could reduce seed yield by 35% and 82% respectively. Surveys of pulse crops over a number of years in Vic and SA showed that up to 15% of crops were infected with PSBMV, with a within crop incidence of up to 5% of plants. In surveys in WA in 1999, PSBMV was found in 42% of field pea crops and the level of virus detected in pea seed stocks was up to 63%. The surveys in WA indicated that PSBMV has a severe effect on seed quality of pulse crops with seed coat symptoms evident on >80% of faba bean, >50% of lathyrus species and field peas and 5% of chickpea seed. At NSW-DPI Tamworth, screening of pea germplasm in the field for resistance to PSBMV has shown that highly susceptible lines may be 100% infected late in the season (Van Leur, unpublished).
Symptoms
The disease is characterized by a mild mottling on the leaves, which is not easily recognizable because of the small size of the lentil leaf.
The affected plants are reduced in growth, which is easily recognized, especially when they are compared with healthy plants. Yield reductions of 15-16% could occur in some different cultivars. Seeds from infected plants occasionally show dark staining on the seed.
Lentils may show no symptoms or there may be chlorosis in new shoots, mottling on leaves, shoot tip necrosis and stunting of plants.
Hosts
The natural host range of PSBMV is limited to the Fabaceae. It infects temperate pulses (chickpea, faba bean, field pea, lentil) other legumes (garden pea, narbon bean) and pastures (lathyrus and vetch). A number of PSBMV pathotypes have been recognised by their ability to infect a number of pea differential genotypes.
Geographic distribution
Worldwide
Biology and transmission
The virus is believed to have spread worldwide through the exchange of infected seed. Seed transmission rates of up to 100% in peas and up to 44% in lentils have been reported. In Victoria, we have detected PSBMV at low levels in some commercial chickpea seedlots (0.4% of seed) and at higher levels in field pea and lentil seedlots (greater than 2% of seed). In the USA, 3% PSBMV infection in pea seedlots and 32-40% in lentil seedlots have been reported. At ICARDA, PSBMV was found to be transmitted through lentil seeds at rates of up to 44%. PSBMV is also transmitted in a non-persistent manner by more than 20 aphid species and by mechanical means. The most efficient vector is the pea aphid (Acyrthosiphon pisum). The other species are as follows: A. pelargoni, A. sesbaniae, Aphis craccivora, A. fabae, A. gossypii, A. nasturtii, Aulacorthum circumflexum, A. solani, Brevicoryne brassicae, Cryptomyzus ribis, Dactynotus escalantii, Macrosiphum avenae, M. euphorbiae, M. pisi, M. rosae, Metopolophium dirhodum, Myzus persicae, Ovatus crataegarius, Phorodon cannabis, Rhopalosiphum padi and Semiaphis dauci. In Victorian pulse crop surveys, we have found cowpea aphid (Aphis craccivora), foxglove aphid (Aulacorthum solani), and green peach aphid (Myzus persicae), which are all vectors of PSBMV.
Detection/indexing method in place at the CGIAR Center at ICARDA
- (TBIA) Tissue Blot ImmunoAssay test, Enzyme-linked immunosorbent assay (ELISA)
Treatment/control
- Seed is considered to be the main source of PSBMV, therefore sowing virus tested seed is the most effective way of controlling this virus. Commercial seed tests are available. Chemical control of aphids is not an effective method for controlling non-persistently transmitted viruses, such as PSBMV. Pulse crops should be sown away from other legumes to minimise the spread of PSBMV.
Procedure followed at the centers in case of positive test
- Destroying seed, producing healthy seed under controlled conditions
References and further reading
http://www.icarda.org/Publications/Field_Guides/Lentil/Lent7.Html
Scientific Name
Broad bean Mottle Virus (BBMV)
Significance
The virus was seen as being of minor importance until reports of its widespread occurrence began to appear in the 1970s and 1980s.
Symptoms
Three quarters of the plants displayed leaf symptoms, many were dwarfed and produced few or no flowers. Infected plants were in concentric patches, BBMV can affect seed quality by causing necrosis and shrivelling of the seed. Seed transmission rates in legumes are low and range from 0.1% to 1.4%.
Hosts
Under experimental conditions susceptibility to infection by virus is found in several families. Susceptible host species are found in the Family Amaranthaceae, Chenopodiaceae, Leguminosae-Papilionoideae, Solanaceae. The following species were susceptible to experimental virus infection: Chenopodium album, Chenopodium amaranticolor, Chenopodium hybridum, Chenopodium quinoa, Cyamopsis tetragonoloba, Glycine max, Gomphrena globosa, Lathyrus odoratus, Lens culinaris, Lupinus albus, Medicago sativa, Melilotus albus, Nicotiana clevelandii, Phaseolus vulgaris, Pisum sativum, Trifolium hybridum, Trifolium incarnatum, Trifolium pratense, Trifolium repens, Trifolium subterraneum, Vicia faba, Vicia villosa.
Geographic distribution
BBMV was reported in faba bean crops in Portugal, Sudan, Morocco, and Algeria. then undertook a regional survey and found BBMV in faba bean crops in Egypt, Morocco, Sudan, Syria and Tunisia. Fortass and Bos (1991) surveyed faba bean crops in Morocco and found that luteoviruses and BBMV were the most prevalent viruses.
Biology and transmission
BBMV survives between growing seasons of the primary pulse host in an alternative host or in infected seed. Due to the fact that the natural host range includes at least four families and includes many food legumes and non-legume wild hosts, it may survive in a range of alternative hosts.
Virus is transmitted by a vector. Virus is transmitted by mechanical inoculation; transmitted by grafting.
Virus is transmitted by arthropods, by insects of the order Coleoptera; Acalymma trivittata, Colaspis flavida, Diabrotica undecimpunctata, Sitona lineata.
Detection/indexing method in place at the CGIAR Center at ICARDA
- Tissue blot immunoassay (TBIA)
Treatment/control
- Seed is considered to be the main source of BBMV, therefore sowing virus tested seed is the most effective way of controlling this virus. Commercial seed tests are available. Chemical control of insects is not an effective method for controlling virus.
Procedure followed at the centers in case of positive test
- Destroying seed, producing healthy seed under controlled conditions
References and further reading
http://www.padil.gov.au/pbt/index.php?q=node/46&pbtID=152
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.010.0.03.002.htm
Fungi - lentil
Contributors to this page: ICARDA, Aleppo, Syria (Siham Asaad, Abdulrahman Moukahal).
Ascochyta blight of lentil, leaf blight, pod spot
Scientific name
Didymella lentis Didymella lentis W.J. Kaiser, B.C. Wang & J.D. Rogers (anamorph Ascochyta lentis Vassiljevsky)
Significance
Ascochyta blight has the potential to occur in all areas where lentils are grown. Serious yield losses due to the disease are uncommon, but pod infection by the pathogen can discolor seed, which can reduce the market value of the crop significantly. This is particularly important in green lentils grown for the whole seed market, which requires high seed quality.
Symptoms
Leaf lesions: Ascochyta blight relies on rain to spread spores from infested debris on the soil surface onto the newly planted lentils. Symptoms may develop after a period of rainfall while the crop is in the vegetative growth stage, during flowering, or even later. The first symptoms appear on leaflets at the bottom of the canopy. Young lesions are small, brown dots. These later expand to 3-6 mm in length and turn tan-colored or brown, sometimes with a dark margin. Pycnidia containing the next generation of spores form in the lesions. Young pycnidia are reddish brown and turn black as they get older. Heavily-infected leaflets or groups of leaves may be completely brown and eventually drop to the ground.
Stem lesions: Ascochyta blight lesions on stems are brown and of variable size. They may girdle the stem, thereby disrupting the epidermal and cortical tissue., The vascular tissues that transport water and nutrients are rarely affected by Ascochyta blight, so, although severe attacks may cause some branches to wilt, entire plants are not killed (in contrast to anthracnose, which penetrates deeper into the stem).
Infection of flowers, pods and seed: Attack of peduncles results in abortion of the flower. Buds or flowers may also be infected. Lesions on pods are a common symptom. The fungus can grow through the pod wall into the seed. Some infected seeds show symptoms such as shriveling and brownish-black discoloration; others have no visible symptoms.
Hosts
Lentil
Geographic distribution
Cosmopolitan
Biology and transmission
The pathogen survives in infected seed and in infected stubble/crop debris. Sowing infected seed can give rise to the appearance of symptoms at the seedling stage. Seed can remain infected for several years. Infected stubble is also an important source of fungal inoculum. Spores are produced on old stubble and are spread to plants by rain splash. Further spread from plant to plant within crops also occurs through rain splash. The development of epidemics is largely determined by environmental conditions, especially levels of moisture. Cool, wet weather with frequent rainfall promotes sporulation, spore dispersal and infection.
Detection/indexing methods used at ICARDA
- Malt extract agar
Treatment /control
- Bravo 500 (50% cholorothalonil, Zeneca Agro) is the only fungicide registered in Canada for control of anthracnose and Ascochyta blight in lentil. The recommended rate is 0.8 - 1.6 L/acre (2.0-4.0 L/hectare) with a maximum of two applications. The water rate is 90-640 L/acre (220-1600 L/hectare). Bravo must be present on the plant surface prior to the onset of fungal infection. It sticks well to the plant surface and resists removal by rain. Fungal resistance to cholorothalonil has not been detected.
Procedures followed in case of positive test at ICARDA
- Severely-infected seed showing disease symptoms is destroyed. Seed not showing symptoms is treated with fungicide.
References and further reading
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Ascochyta blight. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lent4.html. Date accessed 13 April 2010
CSIRO. 2009. [online] Available from URL: http://new.dpi.vic.gov.au/notes/crops-and-pasture/ag1350-ascochyta-blight-of-lentil. Date accessed 13 April 2010
Lindbeck K. 2006. Ascochyta blight of lentil. Agriculture note AG1350. Melbourne: Department of Primary Industries, Victoria, Australia. [online] Available from URL: http://new.dpi.vic.gov.au/notes/crops-and-pasture/ag1350-ascochyta-blight-of-lentil. Date accessed 13 April 2010
PARIDSS (Prairie Agriculture Research Initiative Decision Support System). Lentil diseases: Lentil Ascochyta blight. Information sheet.
Schwartz HF, Gent DH, Mikkelson M, Riesselman J. 2007. Pulse crops: Ascochyta blight of lentil. Information sheet. [online] Available from URL: http://wiki.bugwood.org/uploads/AscochytaBlight-Lentil.pdf. Date accessed 13 April 2010
 |
 |
 |
 |
|
Ascochyta blight of lentil (photos: ICARDA) |
|||
Botrytis gray mold of lentil, lentil Botrytis
Scientific name
Botrytis cinerea Pers.
Other scientific names
Botryotinia fuckeliana (de Bary) Whetzel
Significance
Botrytis gray mold has the potential to occur in all areas where lentils are grown, depending on the season, but is more common in districts with rainfall >400mm. Losses due to the disease can range from minor to very serious, depending on the variety grown, location of the crop, time of infection and amount of spring rainfall. Unprotected crops can lose up to 25 - 30% yield. In addition, seed can be discolored due to pod infection by the pathogens which can further reduce market value of the crop.
Symptoms
All aboveground plant parts of lentil can be affected by botrytis grey mould. Depending on the location of the crop, symptoms may initially appear either on flowers and pods, or lower in the crop canopy. The most damaging symptoms become apparent after the crop has reached canopy closure and a humid microclimate is produced under the crop canopy. The disease appears first as discrete cream colored lesions on lower leaves. These enlarge and coalesce to infect whole leaflets which later senesce and fall to the ground. Unlike Ascochyta blight, no small, black fruiting bodies (called pycnidia) can be seen within the lesions. If conditions remain conducive for disease, that is warm and wet under the crop canopy for at least four days, infection can spread to the lower stems. These lesions will girdle the stem and become covered with a furry layer of grey mould, eventually causing stem death and whole plant death, often occur before the onset of flowering and pod fill. Infection will continue to spread resulting in patches of dead plants within crops. Pods which become infected will be covered in a grey moldy growth, rot, and turn brown when dried out.
 Gray mold (photo: ICARDA) |
Hosts
Broad host range including other legumes and horticultural and ornamental crops.
Geographic distribution
Argentina, Australia, Bangladesh, Canada, Chile, Colombia, India, Myanmar, Nepal, Pakistan, Spain, Syria, Tunisia, Turkey, USA.
Biology and transmission
The fungal pathogens Botrytis cinerea that cause botrytis grey mould can survive as several forms, these include in infected seed, sclerotia in the soil, in old infected residues, and on alternate host plants. Sowing seed that is infected by the botrytis grey mould pathogens can give rise to infected seedlings and the appearance of seedling blight symptoms, which can reduce seedling survival and reduce crop establishment. Infected residues are an important source of fungal inoculum. Spores are produced on old residues and are carried by the wind into new crops where infection can occur.
 Lentil pod infected by Botrytis cinerea. (photo: Department of Primary Industries, Victoria, Australia) |
Detection/indexing methods used:
- at ICARDA - Agar plate test
- at ICRISAT - Agar plate test
Treatment/control
- Use of clean seed is advocated. Seed treatment with fungicides such as benomyl (0.1-0.2%), chlorothalonil (0.2-0.3%), or thiabendazol (0.1-0.2%) is recommended to control seed- borne infection. Two to three year crop rotation and deep plowing should be practiced to reduce soil-borne inoculum.
- Foliar spray using chlorothalonil (2-3 L/ha) in a single application at early bloom to early pod set provides the best protection and increases seed yield.
Procedures followed in case of positive test at ICARDA
- Seed treatment with appropriate funficide
References and further reading
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Gray mold. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lent6.html. Date accessed 13 April 2010
Lindbeck K. 2008. Botrytis grey mould of lentil. Agriculture note AG1257. Melbourne: Department of Primary Industries, Victoria, Australia. [online] Available from URL: http://www.dpi.vic.gov.au/dpi/nreninf.nsf/v/AA398923B804891DCA25746B000FAD89/$file/Botrytis_Grey_Mould_of_Lentil.pdf. Date accessed 13 April 2010
PARIDSS (Prairie Agriculture Research Initiative Decision Support System). Lentil diseases: Lentil Botrytis. Information sheet.
Phoma blight, spring black stem, leaf spot.
Scientific name
Phoma medicaginis Malbr. & Roum. var. medicaginis
Significance
Not significant.
Symptoms
Small, dark brown to black dots on leaves, petioles and stems;
Leaf spots enlarge, coalesce forming irregular blotches;
Infected leaves turn yellow, wither and drop;
Black tissue may appear near base of stems;
Crown and root rot may occur; and
Infected seed pods and seed may discolor and shrivel.
 Spring Black Stem and Leaf Spot (photo: Manitoba Agriculture, Food and Rural Initiatives) |
Hosts
Lentils, other legumes. Most important host is alfalfa.
Geographic distribution
Cosmopolitan
Biology and transmission
Favored by cool and wet conditions; overwinters on dead stems and leaves or in crowns and roots; spores released during periods of wet cool weather; spores spread by rain splash, wind blown or carried by insects; and new shoots exposed through infected residue.
Detection/indexing methods used at ICARDA
- Malt extract agar
Treatment/control
- Use certified seed; use disease resistant cultivars; crop rotation; and crop rotation; and spring burning when severe.
- Application of benomyl seed treatments (0.1 and 0.5% w/w) resulted in only a 4-5 week delay in the onset of Phoma black stem symptoms.
Procedures followed in case of positive test at ICARDA
- Not reported
References and further reading
Manitoba Agriculture, Food and Rural Initiatives. Management of diseases of alfalfa seed. [online] Available from URL: http://www.gov.mb.ca/agriculture/crops/forages/bjb00s28.html. Date accessed 13 April 2010
Scientific names
Glomerella lindemutiana (anamorph Colletotrichum lindemuthianum (Sacc. & Magnus) Briosi & Cavara) and other species such as Colletotrichum truncatum (Schwein.) Andrus & Moore)
Significance
Not significant.
Symptoms
Leaf lesions and premature leaf drop: In most lentil crops, the first symptoms of anthracnose appear before flowering, when the plants have 8 to 12 nodes on the main stem. This is also the time when the first tendrils form, and approximately a week before flowers start to open. If there is a large amount of inoculum in the field the first symptoms may appear earlier. Tan coloured lesions of variable size develop on the lower leaflets and the most severely affected leaflets die and drop to the ground. This premature leaf drop indicates that anthracnose may become a problem, and fungicide application should therefore be considered.
Stem lesions: Lesions on stems develop soon after the appearance of leaf lesions, primarily at the base of the plant. Stem lesions may be small, brownish with a black border, or larger, stretching along the stem. As the season progresses, more and more lesions develop at the stem base, as well as on the upper part of the stems, and many stems are girdled.
Wilt: Anthracnose causes defoliation and stem girdling, which inhibits utilization of water and nutrients, and causes the lentil plants to wilt. As a result, large areas of brown and dying plants can be found in the field.
Hosts
Plant species belonging to the Lens and Vicia families, such as lentil, faba bean, mung bean, and wild vetches.
Geographic distribution
The disease is reported from Bangladesh, Canada, Ethiopia, Morocco, and Syria. It is economically important only in Canada.
Biology and transmission
Small, pinhead sized fungal structures (microsclerotia) form on the infected plant tissue. They may be seen with the unaided eye in the centre of stem lesions or more easily with a hand lens (10-15 x magnification). Each microsclerotia consists of a few hundred cells with thick, black cell walls that protect the fungus from colonization by other microorganisms. Microsclerotia enable the fungus to survive between lentil crops either on the plant debris or free in the soil. They remain viable longer when buried in the soil by tillage than left exposed to weather extremes on the soil surface.
Detection/indexing methods used:
-
at ICARDA
- Agar plate test
- Freezing blotter test
-
at ICRISAT
- Agar plate test
Treatment/control
- Use either certified seed, approved seed, or seed known to have a long disease-free history. The use of disease-free seed is the most important control measure.
- Do not plant beans for at least two years in land that has carried an infected crop.
- Remove diseased plants, where practical, to help check the spread of disease.
- Avoid cultivating and harvesting an affected crop when wet to prevent the spread of spores.
- Do not pack lightly diseased pods as anthracnose can develop during transport.
- Bravo 500 (50% cholorothalonil, Zeneca Agro). The recommended rate is 0.8 - 1.6 L/acre (2.0-4.0 L/hectare) with a maximum of two applications in a season. The water rate is 90-640 L/acre (220-1600 L/hectare). Bravo must be present on the plant surface prior to the onset of fungal infection. It sticks well to the plant surface and resists removal by rain. A second application 10-14 days later may be necessary under wet weather conditions and to protect new growth. Fungal resistance to cholorothalonil has not been detected.
Procedures followed in case of positive test at ICARDA
- Not reported
References and further reading
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Anthracnose. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lent5.Html#_Anthracnose. Date accessed 13 April 2010
North Dakota State University Extension Service. ProCrop 2008: Anthracnose disease in lentils. [online] Available from URL: http://www.ag.ndsu.edu/procrop/lnt/lentan07.htm. Date accessed 13 April 2010
Fusarium wilt, Fusarium root rot, Fusarium vascular wilt of lentil, black root rot
Scientific name
Fusarium oxysporum f.sp. lentis W.L. Gordon
Significance
Fusarium wilt and root rot can cause up to 100% yield losses under heavy infestation, depending on relative humidity, soil moisture, and soil temperature.
Symptoms
Fusarium wilt appears in the field in patches at both seedling and adult stages. Seedling wilt is characterized by sudden drooping followed by drying of leaves and whole seedlings, and apparently healthy-looking roots with reduced proliferation.
Adult wilt symptoms first appear at the flowering to late pod-filling stage. The disease is characterized by sudden drooping of top leaflets of the affected plant, leaflet closure without premature shedding, dull green foliage color followed by wilting of the whole plant or individual branches, an apparently healthy-looking root system with a slight reduction in the lateral roots, as difficult to pull up as the roots of healthy plants, and no internal discoloration of the vascular system in most cases.
Black root rot can appear at any stage of plant growth. The leaves of affected plants show yellowing that progresses from lower to upper leaves. There is rotting of the main and secondary roots, which are characteristically black in color.
Hosts
Lentil
Geographic distribution
Worldwide
Biology and transmission
It can be carried from one field to another on farm equipment, on lentil refuse, and in wind- or waterborne soil. It may be introduced into new areas on the seed. Once introduced into a field it may take two years or more for the fungi to increase in numbers where an appreciable amount of disease is evident. A soil temperature of (23° to 27°C) is most favorable for Fusarium or true wilt, and a slightly higher optimum temperature (27°C) for near wilt. Race 5 of the near wilt fungus, not found in Illinois, infects at lower temperatures. The wilt-producing fungi are soil inhabitants that penetrate the lentil plant through the root hairs and fibrous roots. They grow upward through the stem, often well into the upper branches, in the water conducting tissue (xylem). This process interferes with the passage of water from the roots to the stems, leaves, and pods resulting in yellowing, dwarfing, and wilting of plants. The Fusarium fungi do not reproduce on living plants but produce large numbers of microscopic spores (microconidia, macroconidia, and chlamydospores) in and on dead stems and roots. The spores are splashed or blown about within fields. The spores germinate and the resulting hyphae penetrate the host. The fungi survive in soil for 10 years or longer, in the absence of a lentil crop, as thick-walled chlamydospores. Survival is related to the association of the Fusarium fungi with the roots of nonhost crops. The fungi are also capable of infecting seeds.
Detection/indexing methods used at ICARDA
- Agar plate test
- Freezing blotter test
Treatment/control
- Choose resistant varieties when available. Remove stricken growth and sterilize clippers (one part bleach to 4 parts water) between cuts. Control garden insects, such as cucumber beetles, which are known to spread the disease. Remove all weeds from the garden (many weed species host the disease). The biological fungicide Mycostop will control wilt caused by Fusarium. If the disease persists, it is best to remove the entire plant and solarize the soil before planting again. To solarize the soil, you must leave a clear plastic tarp on the soil surface for 4-6 weeks during the hottest part of the year. Soil solarization will reduce or eliminate many soil inhabiting pests, including nematodes, fungi, insects, weeds and weed seeds.
Procedures followed in case of positive test at ICRISAT
- Not reported.
References and further reading
Bejiga GN, Abou-Zeid W, Suliman SA, Hassanein A. 2001. Managing wilt and root rots of food legumes in the Nile Valley Countries. ICARDA Caravan 15. [online] Available from URL: http://www.icarda.cgiar.org/publications1/caravan/caravan15/managing/managing.html. Date accessed 13 April 2010
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Black root rot. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lent3.Html#_Black. Date accessed 13 April 2010
Beniwal SPS, Baya'a B, Weigand S, Makkouk KH, Saxena MC. 1993. Fusarium wilt. In Field Guide to Lentil Diseases and Insect Pests. Aleppo: ICARDA. [online] Available from URL: http://www.icarda.org/Publications/Field_Guides/Lentil/Lent1.Html. Date accessed 13 April 2010
|
|
|
|
|
|
Fusarium wilt (photos: ICARDA) |
|||
More Articles...
Subcategories
-
main
- Article Count:
- 11
-
Stog
- Article Count:
- 2
-
Stog-rice
- Article Count:
- 7
-
Stog-sorghum
- Article Count:
- 11
-
Stog-common-bean
- Article Count:
- 10
-
stog-forage-legume
- Article Count:
- 10
-
stog-forage-grass
- Article Count:
- 11
-
stog-maize
- Article Count:
- 9
-
stog-chickpea
- Article Count:
- 10
-
stog-millets
- Article Count:
- 12
-
stog-barley
- Article Count:
- 10
-
stog-groundnut
- Article Count:
- 9
-
stog-pigeon-pea
- Article Count:
- 8
-
stog-wheat
- Article Count:
- 10
-
stog-lentil
- Article Count:
- 9
-
stog-cowpea
- Article Count:
- 10
-
stog-faba-bean
- Article Count:
- 9
-
risk management
- Article Count:
- 4
-
decision support tool
- Article Count:
- 3
-
stog-clonal
- Article Count:
- 23
-
developing strategies
- Article Count:
- 4








