CGKB News and events Management strategies
Bacteria - finger millet
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Brown spot; Bacterial brown spot
Scientific name
Pseudomonas syringae pv. syringae.
Other scientific names
Pseudomonas syringae pv. japonica
Importance
High
Significance
The disease is very important throughout the world where finger millet is grown.
Symptoms
Symptoms first appear on the lower surface of the leaves as small, water-soaked spots. The spots enlarge, coalesce, and form larger areas that later become necrotic. The bacteria also enter the vascular tissues of the leaf and spread into the stem. The infected area, which is surrounded by a narrow zone of bright, lemon yellow tissue, turns brown, becomes rapidly necrotic, and through coalescence of several small spots, may produce large dead areas of various shapes. The disease produces identical symptoms on the stems, pods and seeds. In addition, light-cream or silver bacterial exudates are often produced on the lesions under moist conditions.
Hosts
Multilateral hosts like graminacious and leguminacious plants.
Geographic distribution
Pseudomonas syringae pv. syringae is worldwide in distribution.
Distribution of Pseudomonas syringae pathovars into twenty-three O serogroups [online].
Biology and transmission.
Pseudomonas syringae pv. syringae is a rod shaped, gram-negative with polar flagella. It tests negative for arginine dihydrolase and oxidase activity, and forms the polymer levan on sucrose nutrient agar. It is known to secrete the lipodepsinonapeptide plant toxin syringomycin, and it owes its yellow fluorescent appearance when cultured in vitro on King's B medium to production of the siderophore pyoverdin (Goszczynska and Serfontein 1998). Pathogen is seed borne and spreads through the infected seed from season to season (Gaudet and Kokko 1986; Venette et al. 1987).
Detection/indexing methods at ICRISAT
- Pre-export field inspection and agar plate method are used.
Treatment/control
- No suitable seed treatment to eradicate infection
Procedures followed in case of positive test at ICRISAT
- So far not detected at ICRISAT.
References and further reading
Duveiller E, Rudolph K, Fucikovsky L. (eds.). 1997. The Bacterial Diseases of Wheat: Concepts and Methods of Disease Management, Mexico, D.F.: CIMMYT, 78p.
Gaudet DA, Kokko EG. 1986. Seedling disease of sorghum grown in southern Alberta caused by seed borne Pseudomonas syringae pv. syringae. Canadian Journal of Plant Pathology 8: 208-217.
Goszczynska T, Serfontein JJ. 1998. Milk-Tween agar, a semi selective medium for isolation and differentiation of Pseudomonas syringae pv. syringae, Pseudomonas syringae pv. phaseolicola and Xanthomonas axonopodis pv. phaseoli. Journal of Microbiological Methods 32: 65-72.
Mew TW, Misra JK. (eds.). 1994. A Manual of Rice Seed Health Testing. International Rice Research Institute (IRRI), Manila, Philippines, 113pp.
Venette JR, Lampa RS, Albaugh DA, Nayes JB. 1987. Presumptive procedure (dome test) for detection of seed borne bacterial pathogens in dry beans. Plant Disease 71: 984-990.
Scientific name
Xanthomonas vasicola pv. holcicola Elliott.
Other scientific names
Bacterium holcicola, Phytomonas holcicola, Pseudomonas holcicola, Xanthomonas campestris pv. holcicola, Xanthomonas holcicola
Importance to CGIAR centers
High
Significance
Bacterial streak is of minor importance on finger millet.
Symptoms
Symptoms caused by the pathogen are narrow, water-soaked, transparent leaf streaks, 2-3 mm wide by 2-15 mm long, appearing as early as the second leaf stage of the seedling. Lesions soon turn red, become opaque, and at intervals may broaden into somewhat irregularly shaped oval spots with tan centers and narrow red margins. In severe attacks, lesions coalesce to form long irregular streaks and blotches extending across all or much of the leaf blade, with dead tissue bordered by narrow, dark margins between the reddish-brown streaks. Abundant bacterial exudate is produced as light-yellow droplets, which dry to thin white or cream scales (Williams et al. 1978).
Hosts
Panicum miliaceum (millet), Setaria italica (foxtail millet), Sorghum halepense (aleppo grass), Sorghum sudanense (Sudan grass) and Sorghum bicolor (common sorghum).
Geographic distribution
Worldwide
Biology and transmission
Soilborne and infected plant debris transmit the bacteria. Seed transmission of the pathogen is not known so far.
Detection/indexing methods at ICRISAT
- Pre-export field inspection.
Treatment/control
- No seed treatment.
Procedures followed in case of positive test at ICRISAT
- So far not detected.
References and further reading
Williams RJ, Frederiksen RA, Girard JC. 1978. Sorghum and Pearl Millet Disease Identification Handbook. Information Bulletin No. 2. ICRISAT, Patancheru 502324, AP, India. 51pp.
Scientific name
Xanthomonas campestris (Pammel) Dowson
Other scientific names
Xanthomonas pennisiti, Xanthomonas annamalaiensis, Xanthomonas rubrisorghi.
Importance to CGIAR centers
High
Significance
Bacterial leaf streak is of minor importance.
Symptoms
Initial symptoms are narrow, water-soaked, transparent leaf streaks; 2-3 mm wide by 2-15 mm long generally appear from second leaf stage of the seedlings. Lesions soon turn red, become opaque, and at intervals may broaden into somewhat irregularly shaped oval spots with tan centers and narrow red margins. In severe attacks, these coalesce to form long irregular streaks extending across the leaf blade. Abundant bacterial exudates are produced as light-yellow droplets, which dry to thin white or cream scales (Williams et al. 1978).
Hosts
Pearl millet and proso millet (Panicum miliaceum).
Geographic distribution
It is reported from Nigeria and Senegal.
Biology and transmission
Bacterial colonies are yellow and mucoid on the nutrient agar medium. Bacterial cells are aerobic, motile, gram-negative, rod-shaped which differ pathologically, serologically, and by membrane protein patterns from other pathovars of X. campestris. Cells measure 0.45 ´ 2.25 mm and have one polar flagellum. Optimal growth occurs between 26 and 30 oC (Rangaswami et al. 1961).
Detection/indexing methods at ICRISAT
- Pre-export field inspection and agar plate method are used.
Treatment/control
- No seed treatment is available.
Procedures followed in case of positive test at ICRISAT
- So far not detected.
References and further reading
Rangaswami G, Prasad NN, Eswaran KSS. 1961. Two new bacterial diseases of sorghum. Andhra Agricultural Journal 8:269-272.
Williams RJ, Frederiksen RA, Girard JC. 1978. Sorghum and Pearl Millet Disease Identification Handbook. Information Bulletin No. 2. ICRISAT, Patancheru 502324, AP, India. 88pp.
Fungi - finger millet
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Leaf spot; Black kernel
Scientific name
Cochliobolus lunatus RR Nelson & Haasis.
Other scientific names
Acrothecium lunatum, Curvularia lunata, Curvularia lunata var. lunata, Pseudocochliobolus lunatus.
Importance
High
Significance
Less important in finger millet compared to sorghum.
Symptoms
Leaf spots occur on leaves as small, diffuse, and reddish with grayish centers (Shaw 1921).
Hosts
Poaceae (cereals), Oryza sativa (rice), Pennisetum glaucum (pearl millet), Sorghum bicolor (common sorghum), Zea mays (maize), Eleusine coracana (finger millet), Setaria italica (foxtail millet) and several leguminaceous crops.
Geographic distribution
The pathogen is widely distributed in Europe, Asia and Africa.
Biology and transmission
Colonies are effuse, brown, blackish brown or black, hairy or velvety. Conidiophores are solitary or in small groups, simple or branched, straight or flexuous, sometimes geniculate, pale to dark brown, septate, up to 650 µm long, 5-9 µm wide, often swollen at the base to 10-15 µm. Conidia are acropleurogenous, 3-septate, almost always curved at the third cell from the base, which is usually longer and often darker than the others. Cells at each end are sub hyaline or pale brown while intermediate cells are mid to dark brown, smooth, 20-32 ´ 9-15 µm (Anil Kumar et al. 2003).
Detection/indexing methods at ICRISAT
- Pre-export field inspection and blotter test.
Treatment/control
- Not known.
Procedures followed in case of positive test at ICRISAT
- Incineration of the infected plants/seed samples, and rejection of the seed samples.
References and further reading
Anil Kumar TB, Mantur SG, Madhukeshwara SS. 2003. Diseases of finger millet. All India Coordinated Small Millets Improvement Project, Indian Council of Agricultural Research, University of Agricultural Sciences, GKVK, Bangalore, Karnataka, India. 126pp.
Shaw FJF. 1921. Report of the Imperial Mycologist. Scient. Reports Agric. Res. Inst, Pusa, 1920-21, 34-40pp.
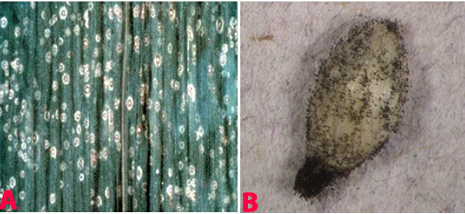 |
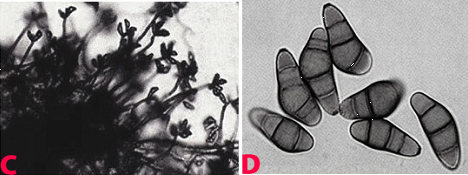 |
|
|
Leaf spot (Cochliobolus lunatus) of finger millet: (A)leaf spots; (B)mycelial growth on seed; (C)conidiophores with conidia and (D)conidia (photos: ICRISAT). |
||
Insects - finger millet
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
Scientific name
Plodia interpunctella Hubner.
Other scientific names
Not known
Importance
Medium
Significance
Indian Meal Moth is a common grain-feeding pest. It can infest a variety of products and is perhaps the most economically important insect pest of processed food. Infestations of P. interpunctella can cause direct product loss and indirect economic costs through pest control costs, quality losses, and consumer complaints (Phillips et al. 2000).
Symptoms
Plodia interpunctella is an external feeder. The larvae continuously spin a silken web both inside and on top of the grain surface, and feed within the web. The webbing contains larval excreta (frass) and exuvia (cast skins), and gives an unpleasant odor to the infested commodity. The infested commodity is sometimes covered on the surface with a thick mat of silken webbing (Mohandass et al. 2007).
Biology and transmission
Female moths lay between 60 and 400 eggs (Lyon 1991) on a grain surface, which are ordinarily smaller than 0.5 mm and not sticky. The eggs hatch in 2 to 14 days. The moth larvae are off-white with brown heads. When these larvae mature, they are usually about 12 mm long. The larval stage lasts from 2 to 41 weeks, depending on the temperature. Adult moths are 8–10 mm long with 16–20 mm wingspans. The outer half of their forewings are bronze, copper, or dark gray in color, while the upper half are yellowish-gray, with a dark band at the intersection between the two. The entire life cycle may range from 30 to 300 days (Mohandass et al. 2007).
 Indian Meal Moth (Plodia interpunctella) of finger millet (photo: ICRISAT). |
Hosts
Arachis hypogaea (groundnut), Oryza sativa (rice), Prunus (stone fruit), stored products (dried stored products), Triticum aestivum (wheat), Zea mays (maize), Avena sativa (oats), Corylus, Helianthus annuus (sunflower), Hordeum vulgare (barley), Juglans regia (Carpathian walnut), Pistacia vera (pistachio), Prunus dulcis (almond) and Theobroma cacao (cocoa).
Geographic distribution
Plodia interpunctella is cosmopolitan in distribution especially in warm climates. In cool temperate countries, it can survive in heated buildings.
Detection/indexing methods
- The primary detection method is through pheromone-based trapping of males (Phillips et al. 2000). The pheromone commonly referred to as ‘‘ZETA’’ was one of the first commercial pheromones for stored-product insects, and the response of males to this pheromone has been well documented.
Treatment/control at ICRISAT
- Fumigation with methyl bromide @ 32 g/m3 for 4 hr followed by seed treatment with chlorpyriphos at 3 g kg-1 seed (Ghanekar et al. 1996; Toews et al. 2006).
Procedures followed in case of positive test at ICRISAT
- Infested samples are rejected in case of positive test.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43
Lyon WF. 2006. Ohio State University Extension Fact Sheet. Entomology. Indianmeal Moth Ohio State University.
Mohandass S, Arthur FH, Zhu KY, Throne JE. 2007. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. Journal of Stored Products Research 43: 302-311.
Phillips TW, Berbert RC, Cuperus G.W. 2000. Post-harvest integrated pest management. In: Francis, F.J. (Ed.), Encyclopedia of Food Science and Technology. 2nd ed. Wiley Inc., New York, pp. 2690–2701.
Toews MD, Campbell JF, Arthur FH. 2006. Temporal dynamics and response to fogging or fumigation of stored-product Coleoptera in a grain processing facility. Journal of Stored Products Research 42:480-498.
Scientific name
Other scientific names
Dinoderus truncatus
Importance
High
Significance
The borer damages grains leading to significant weight loss (5-20%). The quantity of grain dust produced from 6 to 29 g kg-1 grain (Mailafiya 2008).
Symptoms
Adults frequently attack stored seed with intact sheaths by boring into the base of seed. Adults bore into the grains, making neat round holes, and as they tunnel from grain to grain they generate large quantities of grain dust. Adult females lay eggs in chambers bored at right angles to the main tunnels.
 Larger Grain Borer (Prostephanus truncatus) of finger millet (photo: www.agrsci.dk/). |
Hosts
Manihot esculenta (cassava), stored products (dried stored products), Zea mays (maize), Dioscorea (yam) and Triticum aestivum (wheat).
Geographic distribution
Prostephanus truncatus is indigenous in Central America, tropical South America, and the extreme south of the USA as a major pest. It is also distributed in Africa and Europe, but has restricted distribution in India.
Biology
The adult has the typical cylindrical bostrichid shape with body length of 3-4.5 mm. The declivity is flattened and steep and has many small tubercles over its surface. The limits of the declivity, apically and laterally, are marked by a carina. The antennae are 10-segmented and have a loose three-segmented club; the 'stem' of the antenna is slender and clothed with long hairs and the apical club segment is as wide as, or wider than, the preceding segments. The larvae are white, fleshy and sparsely covered with hairs. They are parallel-sided, i.e. they do not taper. The legs are short and the head capsule is small relative to the size of the body.
Detection/indexing methods at ICRISAT
- Pre-export field inspection and dry seed examination using magnifying lens.
Treatment/control
- Not known due to non-detection of the pest.
Procedures followed in case of positive test at ICRISAT
- Incineration of the infested seed samples.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Mailafiya DM, Ayertey JN, Cudjoe AR. 2008.Damage and weight loss potential of Prostephanus truncatus (Horn) Coleoptera: Bostrichidae) on sorghum grain: implication to cereal grain storage in sub-Saharan Africa. International Journal of pure and applied sciences 2: 28-35.
Scientific name
Tribolium castaneum Herbst.
Other scientific names
Not known.
Importance
Medium
Significance
Important in stored finger millet grain in several countries.
Symptoms
Infestation by adult beetles can be readily observed by the tunnels they leave when they move through the flour and other granular food products. Damage is particularly serious in grains such as rice and wheat, which have either been dehusked or processed into other products. When infestation is severe, these products turn grayish-yellow and become moldy, with a pungent odor. Infestation may also be apparent by the appearance of adults on the surface of the grains (Teetes et al. 1983).
 Red Flour Beetle (Tribolium castaneum) of finger millet (photo: ICRISAT). |
Hosts
Arachis hypogaea (groundnut), Avena sativa (oats), Bertholletia excelsa (Brazil nut), Hordeum vulgare (barley), Juglans (walnuts), Lens (lentil), Oryza sativa (rice), Phaseolus (beans), Phaseolus lunatus (lima bean), Pisum sativum (pea), Prunus dulcis (almond), Secale cereale (rye), Triticum (wheat), Triticum spelta (spelt) and Zea mays (maize).
Geographic distribution
Tribolium castaneum is cosmopolitan in distribution. It is found in stored grain, seeds, flour, dried fruits and nuts in worldwide (Teetes et al. 1983).
Biology and transmission
The adult is 2.3-4.4 mm long, rather flat, oblong and chestnut-brown (reddish-brown). It lays about 450 eggs in stored produce. The eggs of are minute, cylindrical and white. The incubation period lasts for 5-12 days. The yellowish-white cylindrical grub is covered with fine hairs. The pupa is naked (without a cocoon), yellowish-white, becoming brown later, and adults emerge in 3-7 days (Teetes et al. 1983).
Detection/indexing methods at ICRISAT
- X-ray radiography is used for suspected samples because it offers a non-destruction of the seed. Dry seed examination using magnifying lens to separate the infested seed.
Treatment/control
- Fumigation of the samples with methyl bromide by 32 gm/m3 for 4 hr followed by treatment with chlorpyriphos 3g kg-1 seed (Ghanekar et al. 1996).
- Procedures in case of positive test at ICRISAT
- Infested samples are rejected.
References and further reading
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Ghanekar AM, Ranga Rao GV, Murthy KS, Surender A, Shaik Babu Saheb. 1996. Seed protectants for healthy exports. Indian Journal of Plant Protection 24: 37-43.
Teetes GL, Seshu Reddy KV, Leuschner K, House LR. 1983. Sorghum insect identification handbook. Information bulletin No.12. Patancheru, A.P., India: International Crops Research Institute for Semi-Arid Tropics. 125pp.
Best practices for the safe transfer of millet germplasm
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Seed health testing protocol at ICRISAT-PQL for import
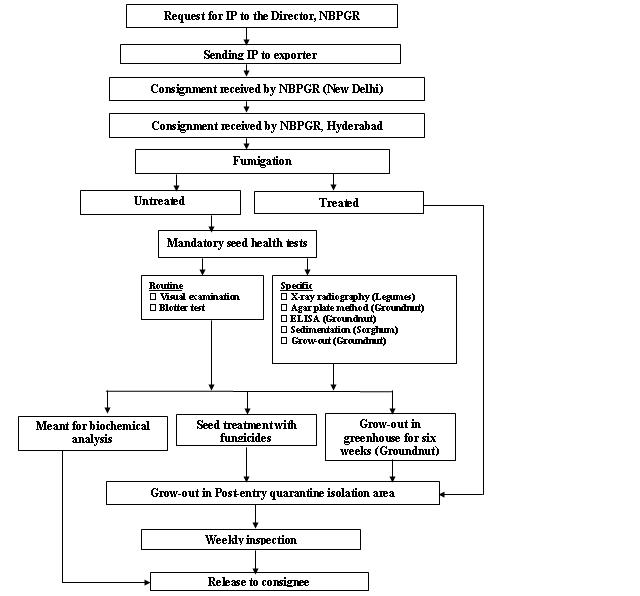
Seed health testing protocol at ICRISAT-PQL for export
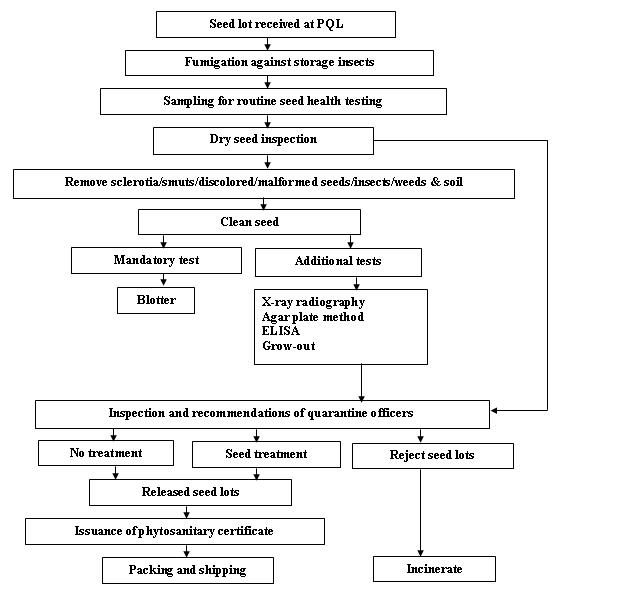
References and further reading
Chakrabarty, SK, K. Anitha, AG Girish, B Sarath Babu, RDVJ Prasad Rao, KS Varaprasad, PK Khetarpal and RP Thakur. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin 69. Rajendranagar 500 030, Andra Pradesh, India. National Bureau of Plant Genetic Resources, Pantacharu 502 324, Andra Pradesh, India. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT).
More Articles...
- Guidelines for the safe transfer of pearl millet germplasm
- Guidelines for the safe transfer of finger millet germplasm
- Insects - pearl millet
- Fungi - pearl millet
- Bacteria - pearl millet
- Safe transfer of barley germplasm
- Import/export of barley germplasm
- Guidelines for safe transfer of barley germplasm
- Viruses - barley
- Bacteria - barley
Subcategories
-
main
- Article Count:
- 11
-
Stog
- Article Count:
- 2
-
Stog-rice
- Article Count:
- 7
-
Stog-sorghum
- Article Count:
- 11
-
Stog-common-bean
- Article Count:
- 10
-
stog-forage-legume
- Article Count:
- 10
-
stog-forage-grass
- Article Count:
- 11
-
stog-maize
- Article Count:
- 9
-
stog-chickpea
- Article Count:
- 10
-
stog-millets
- Article Count:
- 12
-
stog-barley
- Article Count:
- 10
-
stog-groundnut
- Article Count:
- 9
-
stog-pigeon-pea
- Article Count:
- 8
-
stog-wheat
- Article Count:
- 10
-
stog-lentil
- Article Count:
- 9
-
stog-cowpea
- Article Count:
- 10
-
stog-faba-bean
- Article Count:
- 9
-
risk management
- Article Count:
- 4
-
decision support tool
- Article Count:
- 3
-
stog-clonal
- Article Count:
- 23
-
developing strategies
- Article Count:
- 4




