CGKB News and events stog-barley
Safe transfer of barley germplasm
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
ICARDA, as one of the 15 CGIAR Centres, has the mandate for care and maintenance of barley germplasm.
Information is included on:
- Import and export requirements.
- Technical guidelines for the safe movement of germplasm and detection of relevant pathogens and pests.
References and further reading
Mathre, DE (editor). 1997. Compendium of barley diseases. Second edition. APS Press. St Paul, MN. USA. ISBN 0-89054-180-9.
Import/export of barley germplasm
Contributors to this page: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
Barley is a crop under the mandate of ICARDA. It is very important to remember that an International Phytosanitary Certificate is always required for any plant germplasm exchange.
The requirements of countries that request and receive seed from ICARDA Headquarters located in Syria have been collected from the permits granted to ICARDA for exportation of barley experimental seed. The information in this table was updated to May 2009. It indicates specific requirements and the pathogens and pests of which seed should be free when it is sent to a given country.
Please consider that:
- The information received from the same country may vary from a permit to another; therefore the latest has been considered the most valid one.
- Importing seed from another country other than Syria may have other requirements depending on seed origin.
- It is always advisable before sending a shipment to contact the consignee in the recipient country to confirm the information reported in this table.
Guidelines for safe transfer of barley germplasm
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
Technical Guidelines for the Safe Transfer of Germplasm and the Protection of CGIAR Germplasm Banks
Pathogens of quarantine significance for barley (ICARDA)
|
Viruses
|
|
Barley Mosaic Virus
|
|
Barley Stripe Mosaic Virus
|
|
Wheat Streak Mosaic Virus
|
|
Bacteria
|
|
Pseudomonas syringae pv. atrofaciens
|
|
Xanthomonas translucens pv. translucens (ex Jones et al.) Vauterin et al.
|
|
Fungi
|
|
Alternaria spp., Cochliobolus sativus, Fusarium spp., Cladosporium spp.
|
|
Bipolaris sorokiniana (Sacc.) Shoemaker
|
|
Cephalosporium gramineum Nisikado & Ikata in Nisikado et al.
|
|
Claviceps purpurea (Fr.) Tul
|
|
Drechslera graminea (Rabenh.) Shoemaker
|
|
Drechslera teres (Sacc.) Shoemaker
|
|
Drechslera teres f.sp. maculata Smedeg. [spot form]
|
|
Fusarium graminearum Schwabe, [Scab and root rot]
|
|
F. avenaceum (Fr.:Fr.) Sacc. [Scab]
|
|
F. culmorum (Wm. G. Sm.) Sacc. [Scab and root rot]
|
|
Microdochium nivale (Fr.) Samuels & I.C. Hallett (Pers.) Lagerh [Scab]
|
|
Pyrenophora tritici-repentis (Died.) Drechsler
|
|
Rhynchosporium secalis (Oudem.) JJ Davis
|
|
Septoria passerinii Sacc.
|
|
Stagonospora nodorum (Berk.) Castellani and EG Germano
|
|
Ustilago hordei
|
|
Ustilago nuda (C.N. Jensen) Rostr
|
|
Tilletia controversa Kühn
|
|
Insects
|
|
Tribolium confusum Jaquelin Du Val
|
|
Trogoderma granarium Everts
|
|
Rhyzopertha dominica Fabricius
|
|
Sitophilus granarius L.
|
|
Nematodes
|
|
Anguina tritici (Steinbuch) Filipjev
|
|
Weeds
|
|
Cirsium arvense (L.) Scop
|
|
Parasitic weeds
|
Viruses - barley
Contributors to this section: ICARDA, Syria (Siham Asaad, Abdulrahman Moukahal).
|
Contents: |
Barley Stripe Mosaic Virus (BSMV)
Other scientific names
Barley False Stripe Virus, Possibly Barley Yellow Stripe Virus, Barley Mild Stripe Virus, Oat Stripe Mosaic Virus.
Disease name
Barley Stripe Mosaic.
Importance
Seedborne.
Significance
Yield losses are proportional to the level of infection in the seed lot. Heavily infected crops have had yield reductions of up to 25%. The percentage of infected seedlings indicates the level of grain infection.
Symptoms
Yellow to white mosaic, spotting and necrotic stripes.
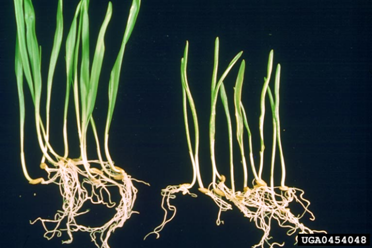 BSMV on the right (source: Bugwood.org) |
Hosts
Natural hosts are members of the Gramineae, but species of Chenopodiaceae and Solanaceae can be experimentally infected.
Geographic distribution
ASIA: Israel, Japan, Jordan, Pakistan; AUSTRALASIA and OCEANIA: Australia (Victoria), EUROPE: Bosnia & Herzegovina, Britain (England), Croatia, Denmark, France, Germany, Kosovo, Macedonia (FYR), Montenegro, Norway, Romania, Russian Federation, Serbia, Slovenia; NORTH AMERICA: Canada, USA; SOUTH AMERICA: Argentina.
Biology and transmission
Transmitted by means not involving a vector. Virus transmitted by mechanical inoculation; transmitted by seed (up to 90-100%); transmitted by pollen to the pollinated plant.
The virus is seedborne and is transferred from plant to plant when the crop leaves rub against one another. Experimentally the virus can be transmitted by pollen, but since barley is self-pollinated this method of spread is generally of no consequence. Infected seeds produce infected plants. Seed from virus-infected plants is generally infected to a 60% level.
Detection/indexing method in place at ICARDA
- Enzyme-linked immunosorbent assay (ELISA), (TBIA) Tissue Blot ImmunoAssay test.
Treatment
- Avoid introducing the disease.
- Use virus-free seed.
- Control volunteer barley. Do not plant barley after barley if the previous barley crop was infected.
Procedure followed at the CGIAR Centres in case of positive testing at ICARDA
- The seed lot is destroyed.
Significance
Not significant.
Symptoms
The Barley Mosaic Viruses are usually first noticed in the winter or early spring as yellow patches in the crop. They causes mosaic and stunting.
 BMV typical symptoms on younger leaves (photo: rothamsted.ac.uk) |
Hosts
Avena sativa, Hordeum vulgare, Triticum aestivum.
Geographic distribution
India and Japan.
Biology and transmission
Virus is transmitted by aphids: Rhopalosiphum maidis and by mechanical inoculation; transmitted by seeds.
Detection/indexing method in place at the CGIAR Centres
- At ICARDA - Not important.
Treatment/control
- Clean the seed. Barley Mosaic Viruses can be effectively controlled using resistant varieties.
References and further reading
http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.079.0.70.003.htm
http://www.apsnet.org/online/common/names/barley.asp
http://www.rothamsted.ac.uk/ppi/staff/mja.html
Seed Health General Publication published by the Centre or the CGIAR.
Wheat Streak Mosaic Virus (WSMV)
Importance
Seedborne.
Significance
Early widespread infection of young wheat plants (approaching 100% infection) is generally associated with greatest yield losses from Wheat Streak Mosaic Virus (WSMV) and can cause complete crop failure, as such crops produce only small amounts of shrivelled grain. Patchy, early infection can also cause substantial yield losses within the affected crop area.
Symptoms
The symptoms of WSMV in wheat appear as pale green streaking on leaves, yellowing of the older leaves (especially towards their tips) and stunted, tufted plant growth. Affected plants become stunted compared to healthy plants when they are infected at an early growth stage (pre-tillering) but this stunting symptom is much less obvious with later infection. Heads on early infected, stunted plants are either sterile and contain no seed, or contain small shrivelled grain. Except for volunteer oats, no symptoms have been seen in Western Australia on WSMV-infected alternative hosts (grasses and barley).
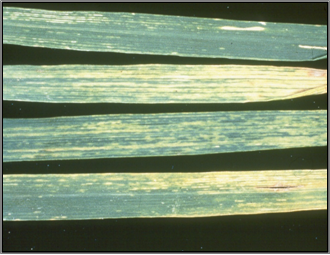 Yellowing of the older leaves (photo: coopext.colostate.edu) |
Hosts
Avena sativa, Hordeum vulgare, Poa compressa, Secale cereale, Triticum aestivum and Zea mays.
Geographic distribution
The virus occurs in the Canada, United States, Jordan, Eastern Europe and Russian Federation.
Biology and transmission
Infections first appear at the margins of a field because of the movement of the virus's mite vector. Infections may occur in the winter, but symptoms often do not appear until the spring temperatures rise to above 10°C.
Transmitted by a vector; a mite; wheat curl mite (WCM) Aceria tulipae; Eriophyidae. The virus is not transmitted congenitally to the progeny of the vector; transmitted by mechanical inoculation; not transmitted by contact between plants; transmitted by seed (very low levels).
Detection/indexing method in place at the CGIAR Centers
- At ICARDA - Not significant.
Treatment
- Serious outbreaks of WSMV can only occur if the mite vector, WCM, is abundant and a source of WSMV is present. Consequently, management of the disease is highly dependent on controlling WCM populations and sources of WSMV. The control options available against WSMV include the following:
- Control the ‘green bridge’ (volunteer crop cereals e.g. wheat, barley, cereal rye, oats and grass weeds) as they harbour both WSMV and WCM. This control needs to be done throughout the paddock (including along the fence line) at least one month before sowing wheat. It needs to be very thorough, involving grazing down to ground level throughout the paddock and early application of herbicide. Neighbouring paddocks should be treated similarly, especially those upwind of the crop. Use of a glyphosphate/araquat mixture provides the most effective control.
- Sow healthy seed stocks of wheat (deemed healthy after seed testing of a representative seed ample).
- Avoid early sowing in virus risk conditions i.e. Sowing directly into a recently sprayed out ‘green bridge’ or sowing of infected seed, as warm seasonal conditions in autumn favour high WCM populations.
Procedure in case of positive test
Not applicable.
References and further reading
http://wheatdoctor.cimmyt.org/index.php?option=com_content&task=view&id=186&Itemid=43
http://www.agroatlas.ru/en/content/diseases/Tritici/Tritici_Wheat_streak_mosaic_rymovirus/
http://www.coopext.colostate.edu/TRA/Agronomy/HighPlains/hpd.figure2.jpg
http://www.dpi.nsw.gov.au/research/updates/issues/july-2006/wsmv-under-scrutiny



 stog-barley
stog-barley
