CGKB News and events stog-forage-grass
Fungi - forage grass
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
Scientific names
Drechslera setarie (S. Ito and Kuribay.) Dastur;
Drechslera sacchari (Butler) Subram. and Jain)
Significance
Several species of Drechslera are important plant pathogens and are associated with symptoms of dark spots on leaves, and root rot of seedlings. Severe forage losses have been recorded in Hawaii and Florida.
Symptoms
Symptoms occur as brown to purple lesions, 1-4 mm in length on leaf blades. Lesions tend to form streaks or eye spots. Leaf spots usually more numerous near the collar area of the leaf blade. Severely affected leaves turn reddish brown, wither and die. Under severe disease pressure, sheath and crown rot may develop resulting in plant death.
Hosts
Cynodon dactylon, Brachiaria spp., Pennisetum purpureum, sugarcane
Geographic distribution
Caribbean, South America, and USA
Biology and transmission
Isolates from sugarcane caused mild symptoms on elephant grass and vice versa.
Detection/indexing method in place at the CGIAR Center
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy. ITSA has approved a detection method of Drechslera oryzae on Oryza sativa (Rice) and is used at GHL [ITSA, 2008].
- at ILRI: None
Treatment/control
- In rice the following fungicides reduced seed infection by Drechslera oryzae: Triphenyltin chloride, Fentin hydroxide, IBP (Kitazin), Zineb and Mancozeb. Two applications of Chlorothalonil (1.5 kg/ha) or Metam-sodium controlled Drechslera oryzae.
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Agarwal VK, Sinclair JB. 1987. Principles of seed pathology. CRC Press, Boca Raton, FL, USA. Volume II Chapter 14 control of seedborne pathogens.
Lenné JM. 1994. Diseases of other pasture grasses. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 169-194. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Weikert-Oliveira RCB. et al. 2002. Genetic variation among pathogens causing "Helminthosporium" diseases of rice, maize and wheat. Fitopatol. bras., vol.27, no.6, p.639-643
References of protocols at EPPO, NAPPO or similar organization
International Seed Testingg Association (ISTA). 2008. International Rules for Seed Testingg Annexe to Chapter 7: Seed Health Testingg Methods. 7-010: Detection of Drechslera oryzae on Oryza sativa (Rice).
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
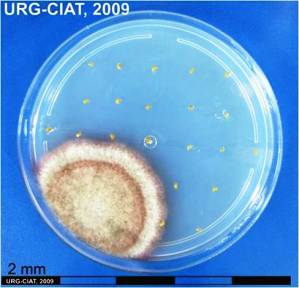
 Drechslera conidia and culture (photos: URG-Virology Unit, CIAT) |
Scientific names
Sphacelia spp. [anamorphs]
Claviceps spp. [Teleomorphs]
Claviceps africana Freder., Mantle & De Milliano 1991
Claviceps paspali F. Stevens & J.G. Hall 1910
Claviceps phalaridis J. Walker 1957
Cepsiclava phalaridis (J. Walker) J. Walker 2004
Other scientific names
Claviceps purpurea (Fr.) Tul. 1883
Significance
Phytosanitary importance. May have nil tolerance. Can render grasses toxic and useless as feed or forage. Inflorescence pathogens (eg., Sphacelia) are readily transmitted by seed. This presents risks in moving germplasm between countries, especially from Africa to other continents. Ergot has considerable potential to be damaging to seed production of Brachiaria spp.
Symptoms
The characteristic symptom of this disease is the dark purplish sclerotia (resting body), that develop in place of healthy seed and protrude from the glume. These ergot sclerotia can be up to four times higher than the normal seed.
The appearance of the sclerotia is preceded by the honey dew stage which appears two to three weeks after flowering. The infected florets exude a yellow, sugary sticky fluid. Insects are attracted to feed on this exudate. Affected heads may appear dirty because of the accumulation of dust and pollen on the sticky honey dew. The ergot of phalaris is not typical, as all florets of infected plants show symptoms. The fungus is systemic within the plant and, in a perennial grass such as phalaris, will persist in its host from season to season.
Hosts
Ergot fungi (Claviceps spp.) are parasites on more than 600 grass species, including forage grasses and leading cereals: wheat, rice, barley, sorghum, oats, rye and millet.
Andropogon gayanus, A. tectorum, Brachiaria spp., Cynodon, Hyparrhenia, Panicum maximum, Paspalum commersonii, P. compressum, P. conjugatum, P. dilatatum (paspalum, Dallas grass), P. distichum (P. vaginatum, salt water couch), P. notatum (Bahia grass), P. orbiculare (ditch millet), P. paspalodes (water couch), P. scrobiculatum, P. urvillei (water couch).
Geographic distribution
Worldwide
Biology and transmission
The genus Claviceps includes much specialized fungi which parasitize only the flowers of specific grasses; no other part of the plant is infected. During infection, the ovary is replaced by a specialized fungal structure called a sphacelium that in time becomes another structure called sclerotium (pseudo-seed). The sclerotium resembles a seed grain but is hard, compact mass of fungal tissue with a thin outer layer (rind). Sclerotia of most Claviceps species are one to four times larger than the host seed. Grasses with small seeds, e.g. Agrostis will yield much smaller sclerotia than larger seeded grasses e.g. Lolium.
When the supply of susceptible flowers is depleted, or the crop is harvested, the fungus somehow has to survive a considerable period of time in the absence of the host. The sclerotia provide one possible means of surviving during this period (winter season in Northern Europe). Once favourable environmental conditions reappear at the start of the next crop season, sclerotia may germinate to produce stalked structures that produce spores, which again may infect the flowers of grass plants.
Claviceps spp. carry-over on alternate hosts and as sclerotia on the soil or mixed with seed.
Cool, wet weather in spring that delays pollination, and thus prolongs flowering, also favours germination of the sclerotia. Stands that tiller and flower unevenly or have a high degree of sterility can be severely affected by ergot.
The germinating sclerotia produce spores which are wind-blown and/or rain splashed onto open florets, where they infect the ovary. Within five days a honey dew is produced containing spores which serve as secondary inoculum. These spores are spread to other florets by contact, rain splash and insects.
Detection/indexing method in place at the CGIAR Center
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
Treatment/control
- Thorough seed cleaning by soaking in 30% salt solution and removing any seeds that float
Procedure followed at the centers in case of positive test
- Reject seeds and repeat regeneration
References and further reading
Seed Health General Publication Published by the Center or CGIAR
Miles JW, Maass BL, do Valle CB; with the collaboration of Kumble V. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
 |
 |
|
|
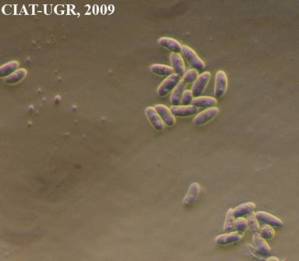
Ergot honeydew and macroconidia [Sphacelia stage] (photos: URG-Virology Unit, CIAT) |
||
Scientific names
Curvularia spp. [anamorphs]
Curvularia cymbopogonis (Dodge) Groves et Skolko
Curvularia penniseti (Mitra) Boedijn
Pseudocochliobolus pallescens Tsuda & Ueyama [Teleomorph]
Other scientific names
Acrothecium penniseti Mitra - Curvularia pallescens Boedijn 1933.
Significance
Widespread in grasses causing reduced yield
Symptoms
Small yellow-brown spots on leaves expand to oblong lesions. Center of lesions change to brown and margins remain yellow. Lesions are more common on leaf margins.
Hosts
Curvularia cymbopogonis: Andropogon spp.
Curvularia pallescens: Axonopus, Bracharia, Coix, Cymbopogon, Cynodon, Dactyloctenium, Digitaria, Echinochloa, Euchlaena, Imperata, Oryza, Panicum, Paspalum, Pennisetum, Rottboellia, Saccharum, Setaria, Sorghum, Sporobolus, Triticum, Zea
Curvularia penniseti: Pennisetum sp. Sorghum bicolor, Triticum; Isolated from Allium, Dolichos and Richardia
Geographic distribution
C. pallescens: Australia,Barbados, Brunei, Burma, Canada, Cuba, Ghana, Hong Kong, India, Indonesia, Jamaica, Kenya, Malaysia, Malawi, Nepal, Nigeria, Pakistan, Papua New Guinea, Peru, Sierra Leone, Singapore, Solomon Islands, Sri Lanka, Sudan, Tanzania, Venezuela, Zimbabwe.
C. penniseti: Australia, India, Nepal, Nigeria, Pakistan, Zimbabwe
Biology and transmission
Other species of Curvularia can be isolated from pearl millet including: Curcularia lunata (Wakker) Boed. A toxin produced by the pathogen is related to host and cultivar specificity; Curvularia geniculata (Tracy & Earle) Boed.
No information is available to indicate if symptoms caused by other species of Curvularia differ from symptoms caused by Culvularia penniseti.
Curvularia species are frequently isolated from seed.
Detection/indexing method in place at the CGIAR Center
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: Culture on oatmeal agar, isolation and identification under microscope
Treatment/control
- Seedborne and no treatments reported
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration seed process in field.
References and further reading
- www.wvu.edu/~agexten/ipm/pestprog/educate/3forage.PDF
- http://www.nilgs.affrc.go.jp/db/diseases/contents/de21.htm
Prithiviraj B, Singh UP, Manickam M, Srivastava JS, Ray AB. 1997. Antifúngical activity of bergenin, a constituent of Flueggea microcarpa. Plant Pathology 46:224-228.
Lenné JM. 1994. Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Stylosanthes. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 21-42. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Pratt RG. 2006. Johnsongrass, Yellow Foxtail, and Broadleaf Signalgrass as New Hosts for Six Species of Bipolaris, Curvularia, and Exserohilum Pathogenic to Bermudagrass. Plant Disease 90 (4):528.
Roberts JA, Tredway LP. 2008. First Report of Curvularia Blight of Zoysiagrass Caused by Curvularia lunata in the United States. Plant Disease 92 (1):173.
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
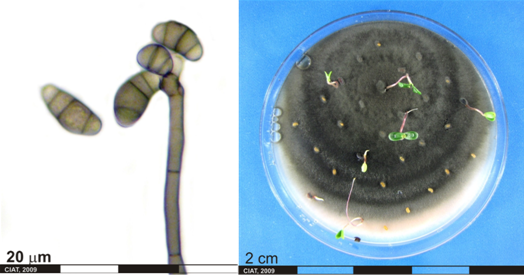 Conidia and culture of Curvularia sp. (photos:CIAT) |
Scientific name
Helminthosporium spp=Exserohilum spp., Bipolaris spp., Drechslera spp.
Significance
Helminthosporium species are causal agents of various diseases that occur in a huge range of plants, including cultivated and wild species.
Symptoms
The genus Helminthosporium includes pathogens whose conidial stages are responsible for serious diseases of rice, corn, grasses, and cereals. In sugarcane is characterized by the formation of eye-shaped lesions, followed by the development of reddish-brown "runners" which extend from the lesion toward the leaf tip.
Hosts
Axonopus spp., Cynodon dactylon, Stylosanthes guianensis, oat, rye, sugar cane, sorghum, Brachiaria decumbens, P. maximum.
Geographic distribution
Cosmopolitan
Biology and transmission
Several members of the genus produce host-specific toxins. These compounds, which are fungal metabolites toxic only to the susceptible host, produce virtually all of the disease symptoms and are critical for pathogenicity of the fungi.
Detection/indexing method in place at the CGIAR Center
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
Treatment/control
- Soak in 0.5% sodium hypochlorite for 10 min
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Artigiani VHF, Bedendo IP. 1995. Patogenicidade de Helminthosporium oryzae a algumas espécies de gramíneas. Sci. agric. (Piracicaba, Braz.), vol.52, no.1
Frederick P, Gary S. 1976. Serinol as an activator of toxin production in attenuated cultures of Helminthosporium sacchari. PNAS 73:4007-4011
Lenné JM. 1994. Diseases of other pasture grasses. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 169-194. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Stylosanthes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 21-42. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/. IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
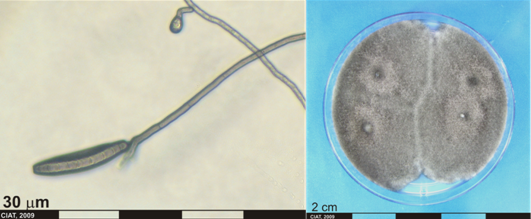 Conidia and culture of Helminthosporium sp. (photos:CIAT) |
Leaf Spots, Black Stem Spotting
Scientific name
Phoma sorghina (Sacc.) Boerema, Dorenb, and van Kest.
Significance
Widespread causing lodging and yield loss
Symptoms
Causes pre-and postemergent damping-off of several legumes. This pathogen is also commonly associated whit necrotic lesions on leaves of Stylosanthes spp. In tropical South America.
Hosts
Desmodium spp., Brachiaria spp., Centrosema spp., Macroptilium atropurpureum, Stylosanthes spp.
Geographic distribution
Nigeria, South America
Biology and transmission
Symptom expression and pycnidial formation required sustained conditions of high relative humidity, so that recognition of the disease may be difficult under dry conditions.
Detection/indexing method
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: Oatmeal agar culture and identification with microscope
Treatment/control
- Hot water treatment at 500C for 20-30 minutes
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Lenné JM. 1994. Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of other pasture grasses. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 169-194. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Macroptilium atropurpureum. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 77-96. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Stylosanthes. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 21-42. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/. IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
 |
 |
|
|
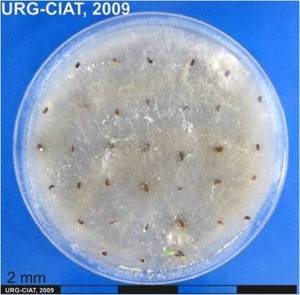
P. sorghina pycnidia and culture (photos: URG-Virology Unit, CIAT) |
||
Scientific names
Pyricularia oryzae Cavara, Pyricularia grisea (Cooke) Sacc., [Anamorphs]
Magnaporthe grisea (T. T. Hebert) Yaegashi & Udagawa. [Teleomorph]
Significance
Pyricularia oryzae is the cause of rice blast, is one of the most important fungal pathogens of rice (Oryza sativa L.) because of its widespread occurrence and destructive nature however this fungi could be found in grass seed.P. grisea is found on grasses as well.
Symptoms
Symptoms are darkened lesions at the panicle neck node and flag leaf collar. Many of the panicles with neck rot were partially filled or blank. The fungus can attack any aerial part of the rice plant, including seeds, in which the fungus may overwinter for several years. There is also latent infection in seedlings by P. oryzae grown under low temperature conditions.
Hosts
Rice (Oryza sativa L.), Lolium perenne, Pennisetum clandestinu, Eleusine indica, Echinochloa colonum
Geographic distribution
Seed transmission of P. oryzae was first reported from Japan and later from other parts of the world.
Biology and transmission
Investigations suggested systemic transmission of the fungus from seeds to seedlings.
Detection/indexing method
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
Treatment/control
- None
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in field.
References and further reading
Greer CA, Scardaci SC, Webster RK. 1997. First Report of Rice Blast Caused by Pyricularia grisea in California. Plant Disease 81 (9):1094.
Lenné JM. 1994. Diseases of other pasture grasses. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 169-194. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Manandhar HK. 1996. Rice blast disease: Seed transmission and induced resistance. Ph.D. thesis. The Royal Veterinary and Agricultural University, Copenhagen. Cited in: Manandhar, H. K., H. J. Lyngs Jorgensen, V. Smedegaard-Petersen, and S. B. Mathur. 1998. Seedborne Infection of Rice by Pyricularia oryzae and Its Transmission to Seedlings. Plant Disease 82 (10):1093.
Manandhar HK, Lyngs Jorgensen HJ, Smedegaard-Petersen V, Mathur SB. 1998. Seedborne Infection of Rice by Pyricularia oryzae and Its Transmission to Seedlings. Plant Disease 82 (10):1093.
Radjacommare R, Ramanathan AK, Sible GV, Harish S, Samiyappan R. 2004. Purification and anti-fungal activity of chitinase against Pyricularia grisea in finger millet. World Journal of Microbiology & Biotechnology 20: 251–256.
Rice pathology. In: Centro Internacional de Agricultura Tropical. Integrated Pest and Disease Management in Major Agroecosystems: Project PE-1: Summary Annual Report 2003. CIAT, Cali, CO. p. 199-206.
Wong FP, Gelernter W, Stowell L. 2005. First Report of Pyricularia grisea Causing Gray Leaf Spot on Kikuyugrass (Pennisetum clandestinum) in the United States. Plant Disease 89 (4):433.
Wong FP, de la Cerda KA. 2006. First Report of Pyricularia grisea (Gray Leaf Spot) on Perennial Ryegrass (Lolium perenne) in Nevada. Plant Disease 90 (5):683.
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds.). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food andAgriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
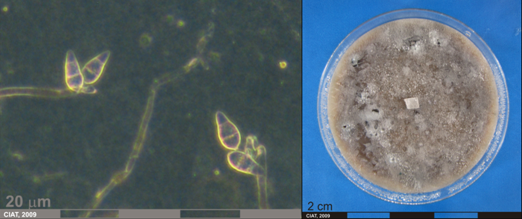 Conidia and culture of Pyricularia oryzae (photos:CIAT) |
Rhizoctonia root rot, Rhizoctonia foliar blight
Scientific names
Rhizoctonia solani Kühn [Anamorph]
Thanatephorus cucumeris ( Frank) Donk [Teleomorph]
Significance
Minor
Symptoms
Both pre-emergent and post-emergence seedling death can occur with this disease. Pre-emergence symptoms are seed decay and are often not visible in the field. Post-emergence symptoms on seedlings will be the appearance of brown to reddish lesions on stems and roots just below the soil line. These reddish brown lesions may become sunken and girdle the stems and kill the plant. Plants may often appear stunted and unhealthy throughout the season or, less commonly, will die. Often the stand will appear uneven because of stunted plants. On older plants, the pathogen causes a reddish brown dry cortical root rot that may extend into the base of the stem. Later in the season, infections at the base of the plant (cortical rot) may result in plants snapping off during high winds. Root rot can greatly reduce nodulation. Foliar symptoms may include yellowing or wilting of leaves.
Damping-off of legume seedlings; web blight resulting in premature defoliation; leaf rust
Foliar blight appears initially as water-soaked patches in the foliage canopy. Profuse growth of fungal mycelium throughout the foliage causes mats of leaves to be stuck together with mycelia strands. Sclerotia are common on blighted leaves. Under prolonged humidity, the patches may extend, causing considerable rotting and death of foliage. Death leaves and petioles are covered and matted together by greyish brown to white fungal mycelium.
Hosts
Brachiaria humidicola, Brachiaria dictyoneura, Brachiaria brizantha
Zea mays
Geographic distribution
USA
This disease has been recorded in Central and South America, Florida (USA), Malaysia, Papua New Guinea, Solomon Islands, Zambia and globally throughout the tropics.
Biology and transmission
Rhizoctonia solani can be found in most soils and survives as sclerotia (very resistant fungal survival structures) in soil.
Rhizoctonia root rot is usually common in warm, moist sandy soils. Stresses in plants which have been observed to favor disease development include herbicide injury, soil insect damage, hail, sandblasting and soybean cyst nematode feeding.
Damage from Rhizoctonia is commonly observed in areas when there is a long history of soybean production with close rotations or during weather conditions not favourable for seed germination and rapid growth of seedlings.
Initiation and development of foliar blight from foci at the beginning on the wet season are favored by high relative humidity, frequent prolonged rain and moderately high temperatures. In Central and South America, foliar blight is most severe in regions with greater than 1500 mm mean annual precipitation. The fungus probably spreads from perennial plants in the pasture and plants debris in the soil. The pathogen complex is primarily soil-borne. Sclerotia readily survive in soil for several years and are disseminated by wind, rain and animals.
Detection/indexing method
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
- at ICARDA: PDA
Treatment/control
- Fungicides can be used
Procedure followed at the centers in case of positive test
- Crop rotation, fungucid seed treatments
- Reject accession and new regeneration started in field.
References and further reading
- http://pdc.unl.edu/agriculturecrops/soybean/rhizoctonia
- http://www.fao.org/ag/AGP/AGPC/doc/Publicat/Gutt-shel/x5556e0s.htm
Lenné JM. 1994. Diseases of Aeschynomene. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 97-107. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI: Oxon, GB.
Lenné JM. 1994. Diseases of Centrosema. In 'Diseases of tropical pasture plants.' (Eds JM Lenné and P Trutmann) pp. 43-60. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Desmodium. In 'Diseases of tropical pasture plants'. (Eds JM Lenné and P Trutmann) pp. 61-76. (CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.).
Lenné JM. 1994. Diseases of Macroptilium atropurpureum. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 77-96. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Seed Health General Publication Published by the Center or CGIAR
Frison EA, Bos L, Hamilton RI, Mathur SB, Taylor JD. (eds). 1990. FAO/IBPGR Technical Guidelines for the Safe Movement of Legume Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board for Plant Genetic Resources, Rome.
 |
 |
|
|
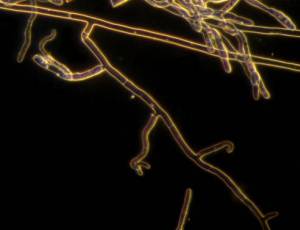
R. solani hyphae and culture (photos: URG-Virology Unit, CIAT) |
||
Scientific names
Tiletia aryesii Berk.
Significance
Smut substantially reduces seed production in tropical America. Although seed production may be greatly reduced, plant vigor does not appear to suffer.
Symptoms
Spikelets of affected inflorescences are open, swollen and filled with semiagglutinate, greyish spore masses, which are released in a grey could when inflorescence are shaken.
Hosts
Panicum maximum, Panicum spp., Setaria spp.
Geographic distribution
Africa: Cameroon, Congo, Ethiopia, Ghana, Ivory Coast, Kenya, Madagascar, Malawi, Mali, Mauritius, Mozambique, Nigeria, Sierra Leone, South Africa, Sudan, Tanzania, Togo, Uganda, Zaire, Zambia, Zimbabwe; Asia: Sri Lanka; Central America: Costa Rica; South America: Brazil, Colombia
Biology and transmission
As spores are wind-borne, infection of opened flowers readily occurs. Naturalized plants around pastures are a major source of infection for seed crops. Smut is also seed-borne.
Detection/indexing method
- at CIAT: PDA Test and direct visualization in Stereomicroscopy and Microscopy.
- at ILRI: None
Treatment/control
- Many seeds are destroyed by smut, but if any are harvested they can be treated with Copper Oxide, Carboxin and Benomyl or hot water to kill spores.
Procedure followed at the centers in case of positive test
Reject accession and new regeneration started in field.
References and further reading
Lenné JM. 1994. Diseases of other pasture grasses. In Diseases of tropical pasture plants. Eds JM Lenné and P Trutmann pp. 169-194. CAB International; Centro Internacional de Agricultura Tropical (CIAT); Natural Resources Institute (NRI): Oxon, GB.
Mordue JEM, IMI Descriptions of Fungi and Bacteria Tilletia ayresii. CABI Bioscience, Bakeham Lane, Egham, Surrey, TW20 9TY, UK.
Scientific names
Ustilago kamerunensis P. & H. Sydow
Significance
Major pest and disease threat to Napier grass.
Affects adversely the small-scale diary industry.
Symptoms
Napier grass, turning vigorous, impenetrable clumps of valuable livestock feed into thin, shrivelled stems.
Presence of fungal spores in the inflorescence.
Hosts
Pennisetum purpureum
Geographic distribution
Africa: Kenya
Biology and transmission
The main means of spread is through transport of infected planting material.
Smut disease causes serious loss of biomass of fodder crops.
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: Potato dextrose media, isolation and identification
Treatment/control
- Napier grass rarely produces sseeds and the mycelia remain in the cuttings or in the embryo of nay seeds. If seeds are harvested, they can be treated with systematic fungicides or hot water.
Procedure followed at the centers in case of positive test
- Rogue and burn all infected plant tissue.
References and further reading
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Nematodes - forage grass
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
|
Contents: |
Stem and bulb nematode infection
Scientific name
Ditylenchus dipsaci (Kühn) Filipjev
Significance
A major pest in temperate climates and high-altitude regions of the tropics and subtropics.
Symptoms
Plants become distorted and stunted; infected tissues are spongy; damage can predispose plants to other problems
Field shows irregular areas of sparse growth
Hosts
More than 400 host plants have been described for D. dipsaci. The species is subdivided in races.
Oat race: polyphagous on cereals, most grains, rye, corn, and oats.
Geographic distribution
Cosmopolitan, except tropical lowlands; temperate regions where it is one of the most devastating plant parasites; USA
Biology and transmission
Nematode is a migratory endoparasite. At the beginning of the crop season, 4th-stage juvenile enters young tissues, especially seedlings when below the soil surface. Feeding breaks down middle lamellae; nematode probably secretes a pectinase enzyme; plant parts become ‘crisp’ and are easily broken. Migration on plant parts above ground requires free water, and may occur after rain or sprinkler irrigation. Nematode enters through stomata or by direct penetration.
Cardinal temperatures for nematode activity and infection are 10oC - 22oC -30oC. In soil, they survive as fourth stage larvae at temperatures not exceeding 35oC.
Infestation occurs readily in heavier soils and during times of high rainfall or in sprinkler-irrigated areas.
Nematode is spread around field by equipment, irrigation; spreads readily in tail water.
Detection/indexing method
- at CIAT: Washing roots and microscopic identification
- at ILRI: Waching roots and microscopic identification
Treatment/control
- Systemic insecticides and hot water treatment
Procedure followed at the centers in case of positive test:
- Reject accession and new regeneration started in clean field area.
References and further reading
Diekmann M. 1997. FAO/IBPGR Technical guidelines for the safe movement of Germplasm. No. 18. Allium spp. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Root-knot nematode infestation
Scientific name
Meloidogyne spp.
Significance
Quarantine importance for vegetative cuttings due to limited distribution and severe damage caused on plant quality and quantity.
Symptoms
Root knot nematode infestation includes wilting, loss of vigor, yellowing and other symptoms similar to a lack of water or nutrients. Plants often wilt during the hottest part of the day, even with adequate soil moisture, and leaves may turn yellow. Fewer and smaller leaves and fruits are produced, and plants heavily infested early in the season may die.
Root knot nematodes usually cause distinctive swellings, called galls, on the roots of affected plants. Infestations of these nematodes are fairly easy to recognize by digging up a few plants with symptoms, washing or gently tapping the soil from the roots, examining the roots for galls. The nematodes feed and develop within the galls, which may grow to as large as 1 inch in diameter on some plants but are usually much smaller. The water- and nutrient-conducting abilities of the roots are damaged by the formation of the galls. Galls may crack or split open, especially on the roots of vegetable plants, allowing the entry of soil-borne, disease-causing microorganisms.
Root knot nematode galls are true swellings and cannot be rubbed off the roots, as can the beneficial nitrogen-fixing nodules on the roots of legumes.
Hosts
Root knot nematodes have a broad host range of more than 200 reported plants.
Geographic distribution
Southeast Asia, South America, USA
Biology and transmission
The root-knot nematodes (Meloidogyne spp.) form easily recognized galls on the roots. Galls result from growth of plant tissues around juvenile nematodes which feed near the center of the root. Root-knot gall tissue is firm without a hollow center, and is an integral part of the root; removing a root-knot gall from a root tears root tissue.
They are difficult to control and can be spread easily from garden to garden in soil (for example, on tools, boots, etc.) and plant parts.
Certain marigolds (Tagetes sp.) suppress root knot nematodes. The effect of marigolds is greatest when they are grown as a solid planting for an entire season. When grown along with annual vegetables or under trees or vines (intercropping), nematode control is usually not very good. As with other cultural control methods, nematode populations will rapidly increase as soon as susceptible crops are grown.
Root knot nematodes may feed on the roots of grasses and certain legumes without causing galling.
Damage is most serious in warm, irrigated, sandy soils.
Detection/indexing method
- at CIAT: Washing roots and microscopic identification
- at ILRI: Not applicable
Treatment/control
- Certain marigolds (Tagetes sp.) suppress root knot nematodes. The effect of marigolds is greatest when they are grown as a solid planting for an entire season. When grown along with annual vegetables or under trees or vines (intercropping), nematode control is usually not very good. As with other cultural control methods, nematode populations will rapidly increase as soon as susceptible crops are grown.
- Systemic insecticides and hot water treatment.
Procedure followed at the centers in case of positive test
- Reject accession and new regeneration started in clean field area.
References and further reading
http://plantclinic.cornell.edu/FactSheets/nematodes
http://www.ipm.ucdavis.edu/PMG/PESTNOTES
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Best practices for safe transfer of forage grass germplasm
Contributors to this page: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
|
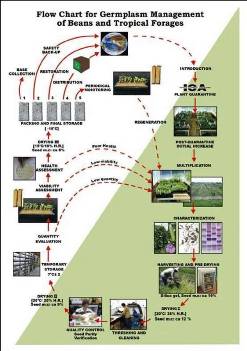
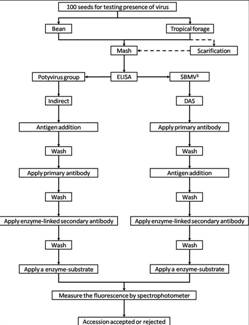
Flowchart 1.Germplasm management of Beans and Tropical Forages (Click to increase the size) |
The agreement between the CIAT and the International Treaty on Plant Genetic Resources for Food and Agriculture implies of the Genetic Resources Program (GRP) the conservation on behalf of the countries of 65,505 materials for 745 plant species of bean, cassava and tropical pastures as the biological patrimony of 141 countries. This responsibility of conservation is associated with the distribution of samples of this patrimony (517,916 samples distributed to 136 countries to the date) according to a regulation defined by the countries inside the Agreement, and phytosanitary procedure established by the Colombian Agricultural Institute.
The job of the GRP is to safeguard the genetic diversity of beans, cassava, forages, and their wild relatives through a mix of conservation methods, both in situ (in a natural outdoor habitat) and ex situ (in the controlled environment of a gene bank). Among the GRP's activities are research to improve conservation methods (including ways to minimize risks to the collections); screening germplasm for diseases and certifying it; duplicating materials of the collection; collecting or otherwise acquiring novel materials; recording passport, characterization and evaluation data for accessions of the collections (See Flowchart 1).
Phytosanitary risks are associated with international movement of germplasm, especially the inadvertent transport of pathogens and pests of quarantine significance. To minimize the phytosanitary risks associated with exchange germplasm and to ensure that it is free from pests and pathogens of quarantine significance; CIAT has implemented regulatory measures and safeguards to complement quarantine guidelines. The process, wich is supervised by the the Agricultural Colombian Institute (ICA), includes the following activities:
- Minimize the risk of accidental introduction of exotic pest and pathogens into Colombia;
- Inspect plant and facilities in screenhouses and grenhouses where the imported germplasm is being incresased;
- Inspect plants in fields and greenhouses where the germplasm intended for international export is produced; and
- Determine the seed health status of germplasm for international export.
The responsibility over the areas dealing with animal and plant health, with regard to international trade, has been bestowed upon the Agricultural Colombian Institute, within regulatory decree 1840 of 1994 “for which (ICA) has the mission of preventing the risk of the entry, spread, and establishment of exotic diseases, those of national sanitary concern, and of chemical risks, and protecting the sanitary quality of animals, plants and products that are exported, to minimize losses in animal-plant production and contribute to the security in foodstuffs”.
ICA has established quarantine procedures to regulate the introduction of plant germplasm and the issuing of phytosanitary certificates that accompany exports. ICA has a Plant Quarantine Station at an altitude of 2600 m (4o 42’ N latitude and 74o 12’W longitude) near Mosquera (Cundinamarca), about 15 km west of Santafé de Bogotá. ICA provides the regulatory mechanism for germplasm exchange following guidelines of the International Plant Protection Convention (IPPC). The recommendations of the IPPC were adopted by Colombian Congress in 1981 and implemented under decrees 501 of 1989.
In order to facilitate the importation and exportation, the ICA has developed the SISPAP, which may accessed through the ICA webpage in which the parties interested in importing and exporting plant products may become aware of the following via internet.
In Colombia, additional guidelines to reduce phytosanitary risks are in place. These safeguards are implemented according to the geographic origin and economic importance of the plant species concerned, and characteristic of potential pathogens and pests. Currently, ICA has a plant quarantine agreement with CIAT establishing which guidelines and safeguards are updated to facilitate germplasm exchange according to national and international requirements. An agreement was signed in 1981 between CIAT and ICA for quarantine procedures to regulate the introductions of germplasm into Colombia. The agreement permits the transit of seed through customs and quarantine stations according to the level of potential risk of introducing pests and diseases no yet reported in Colombia. High risk areas include Africa, Asia, and some European countries. The agreement covers not only crops for wich CIAT has a mandate but also other crops of economic importance to Colombia.
After clearance, materials pass through a step of multiplication in greenhouse stage, and the harvested seeds are subsequently planted in isolated fields. During these two steps, a phytosanitary follow-up is carried out. Plants showing any symptom of fungal, bacterial or viral disease are destroyed.
CIAT facilities for germplasm health testing are designed as a multifunctional laboratory to test seeds and tissues for fungi, bacteria, viruses, and occasionally nematodes and insects (See table below). The purpose of the Germplasm Health Laboratory (GHL) is to ensure that the designated germplasm is kept under the international phytosanitary standards for each crop commodity, and also to ensure that the germplasm distributed by GRP is free of diseases of quarantine importance (listed in the table below).
The ICA Plant Quarantine Officer, stationed at CIAT, carries out field and greenhouses inspections and issues "ICA Phytosanitary Certificate" bases upon those inspections and results obtained by GHL. This document accompanies all out-going germplasm from Colombia (“decrees 1840 of 1994 articule 3”).
The germplasm leaving CIAT and the one used for exchange, conservation, and characterization in the GRP are multiplied in isolated fields under favorable ecological conditions with supervision by ICA quarantine officers. The GRP has seed multiplication sites in Popayan (Cauca) and Santander de Quilichao (Cauca). The harvested seeds are analyzed by GHL prior to shipment abroad or long-term conservation.
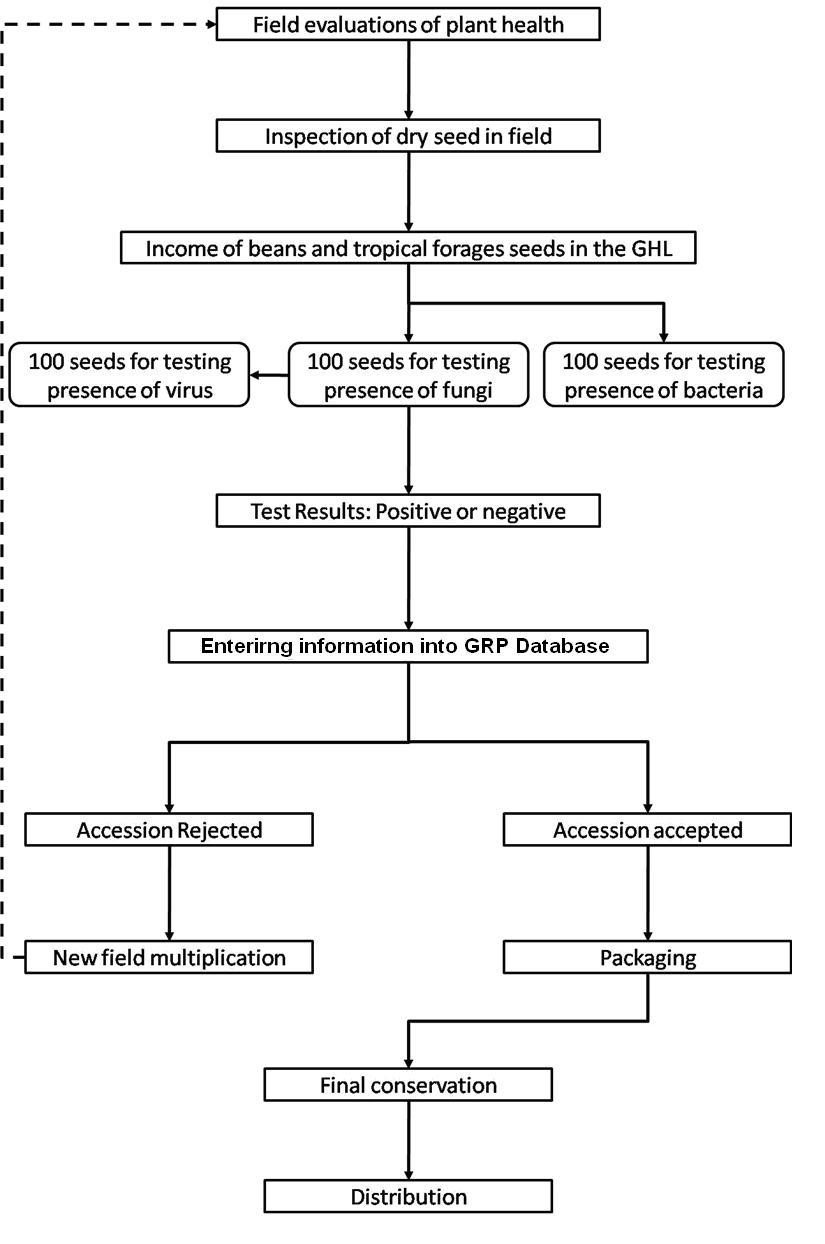
Seed health testing activities include:
- Reception, registration, sampling and storage of incoming material.
- Preparation of working samples for testing.
- Analysis.
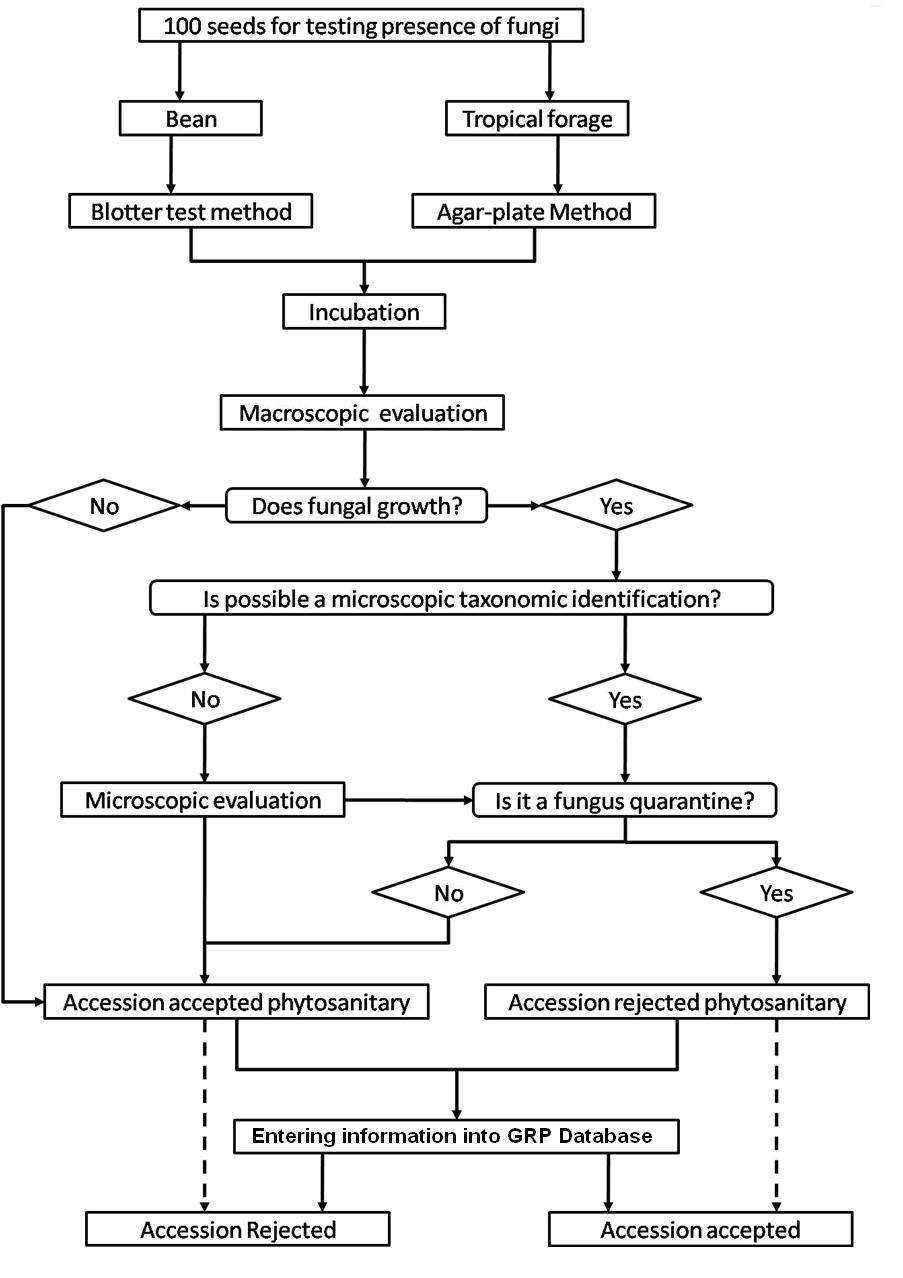
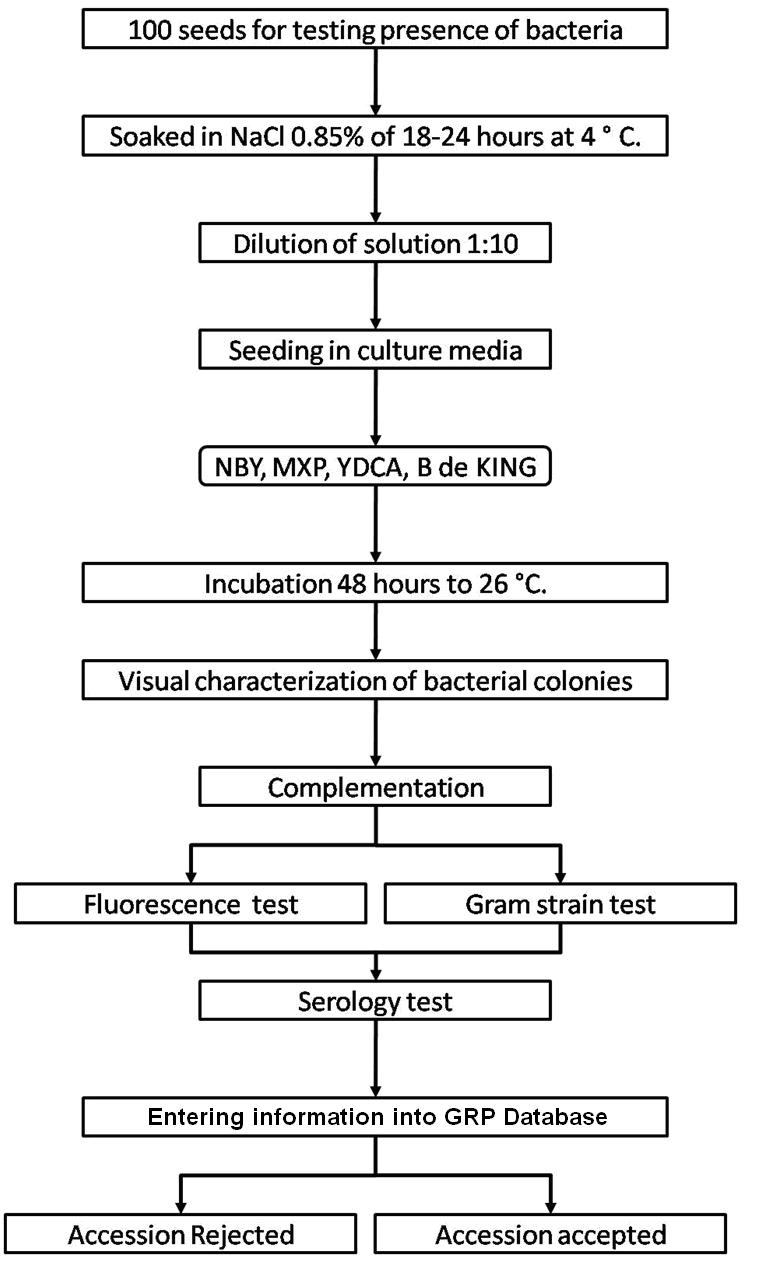
The seed health testing methods used at CIAT for beans and tropical pastures are summarized in The Handbook of Procedures of the Germplasm Health Laboratory (see also Flows charts 2, 3, 4, 5 below, for a quick reference).
To detect pathogens of quarantine significance, the GHL uses the methodologies recommended by CIAT pathologists and virologists (see table below). When a recipient country has additional requirements, the GHL carries out additional tests wherever possible to comply with the specific quarantine regulations of the recipient country. The GHL realizes additional researches in the management and characterization of pathogens of quarantine importance and in the standardization of new methodologies of diagnosis that are more effective and sensitive.
ILRI carries out pathogen detection tests before each regeneration. Seeds are withdrawn from the genebank, scarified and germinated using appropriate conditions for the species. Seedlings are transferred to sterilized compost in seedling trays after germination and grown in a virus screened area until one to three leaf stage. Visual inspection is carried out and seedlings are batch tested in batches of 10 seedlings using TBIA for common grass viruses. Individual seedling tests are performed on any positive batches for confirmation. Clean seedlings are released to the field for regeneration.
If the seedlings are infected with seed borne pathogens and more original seeds are available for a second regeneration, the seedlings are destroyed by incineration. If no original seeds are available, the seedlings are transferred to large pots and retained in a virus screened area for seed production. Since virus transmission is rarely 100%, the seeds are harvested from infected plants and germinated. The seedlings are tested as above to identify clean seedlings for release to the field. If all seeds are infected, thermotherapy and meristem culture are used to eliminate the virus and obtain clean plantlets in in vitro culture. Rooted plantlets can be acclimatized in the greenhouse and these plants used as a source of clean seeds.
Plants in the field are regularly inspected for virus symptoms and samples taken for testing with either TBIA or ELISA for common grass viruses. Some grass species are maintained in the field genebank and also require regular inspection. Young leaves are sampled from Napier grass for diagnosis for phytoplasma associated with Napier grass stunt using NASH. Plants that test positive for virus diseases are rogued from the field.
Field inspection is also carried out for pathogenic fungi and insects and any infection is controlled through field management. Regeneration and field genebank plots are sprayed with fungicides or insecticides at early signs of infection. Regular cutting is done on grass plants in the field genebank to reduce fungal diseases.
ILRI uses a range of different methods to detect pathogens of forage grass germplasm. Click the forage grasses health table for specific species information about health diagnosis methods to detect some of these diseases. Detailed protocols were developed for the detection of:
- Virus (ELISA and TBIA).
- Phytoplasm (NASH).
- Fungi (seed washing technique; blotter test; potato dextrose agar; oatmeal agar).
References and further reading
Cuervo MI, Balcazar MS, Ramirez JL, Medina CA, Debouck D. 2009. Manual de operaciones laboratorio sanidad de germoplasma – unidad de recursos genéticos. GRP, CIAT, Colômbia. 72 pp. Available here
Plant Biosecurity, Biosecurity Australia 2000. Viruses, Phytoplasmas and Spiroplasmas of Clonal Grasses and Their Diagnosis. Consultancy report. Available from: http://www.daff.gov.au/__data/assets/word_doc/0020/24761/consul_rpt_clonal.doc. Date accessed: 30 March 2010.
Viruses - forage grass
Contributors to this page are: CIAT, Colombia (Maritza Cuervo, Cesar Medina, Jose Luis Ramirez, Socorro Balcazar, Josefina Martinez, Daniel Debouck); ILRI, Ethiopia (Jean Hanson, Janice Proud, Juvy Cantrell).
|
Contents: |
Scientific name
Digitaria striate mosaic virus (DiSMV or DSMV)
Significance
Minor significance for quarantine because widespread. It can affect several important forage grasses, maize and wheat.
Symptoms
Chlorotic striations and stripes
Narrow white streaks or local lesions, usually 1-3 mm long; longer wider chlorotic often yellow local lesions with diffuse margin; narrow, short, white or chlorotic streaks or local lesions with some showing diffuse edges, but most local lesions clearly defined; chlorotic streaks and local lesions, severe deformation of pant.
Hosts
Avena sativa, Brachiaria subquadripara (syn. B. miliiformis), Chloris gayana, Digitaria decumbens (D. eriantha), Digitaria ciliaris, Digitaria sanguinalis, Digitaria setigera, Dinebra retroflexa, Echinochloa colona, Eleusine indica, Hordeum vulgare, Lolium multiflorum, Sorghum bicolor, Zea mays
Geographic distribution
Worldwide
Queensland (Australia), India (Maharashtra State)
Biology and transmission
Transmitted in nature by the planthopper Sogatella kalophon but not by seeds or mechanical means.
Vetor specificity and host preference should limit the spread of DSV.
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- None, not seed borne
Procedure followed at the centers in case of positive test
- For low infection, rougue the field and burn infected plants. For high infection produce seeds in a screenhouse to obtain disease free seeds to re-establich plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Viruses of Plants. Description and Lists from the VIDE Database. CAB International, UK. 1484 pp.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecht. 800 pp.
Seed Health General Publication by the Center or CGIAR
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Scientific name
Elephant grass mosaic virus (EGMV)
Significance
Although EGMV has been found only in elephant grass, experimental transmission to corn and sorghum indicates that the virus maybe of economic importance.
Symptoms
Mosaic in leaves; chlorotic spots and streaks
Hosts
Andropogon schoenanthus, Avena sativa, Chenopodium amaranticolor, C. quinoa, Gomphrena, globosa, Hordeum vulgare, Oryza sativa, Panicum compressum, P. maximum, Panicum maximum, Pennisetum purpureum, Secale cereale, Stenotaphrum secundatum, Sorghum bicolor, Triticum aestivum, Zea mays
Geographic distribution
East Africa, Brazil
Biology and transmission
A virus isolated from leaves of elephant grass (Pennisetum purpureum). It was mechanically transmitted to a few cultivars of Zea mays and Sorghum bicolor, but other test plants including elephant grass could not be infected.
It was not transmitted by Myzus persicae Sulz. and Rhopalosiphum maidis Fitch.
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection check for disease free suttings to re-establish plots.
References and further reading
http://cat.inist.fr/?aModele=afficheN&cpsidt=3793303
http://www.apsnet.org/pd/PDFS/1993/PlantDisease77n07_726.PDF/
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Marins CRF, Kitajima EW. 1993. A unique virus isolated from elephant grass. Plant Disease. 77(7): 726-729.
Scientific name
Guineagrass mosaic virus (GMV)
Significance
Causes reduced yields in grasses in tropical areas and not seed borne but can affect maize.
Symptoms
Systematically infected plants show characteristic rhomboid or eye-shaped lesions on infected leaves. As the disease progresses, various mosaic patterns and chlorotic patches develop, causing early leaf senescence. As long as infected plants are maintained under favorable conditions, the disease does not cause significant damage.
Light-green or yellow mosaic
Hosts
Avena sativa, Brachiaria decumbens, B. deflexa, B. dictyoneura, B. humidicola, B. jubata, B. ruziziensis, Dactylis glomerata, Digitaria sanguinalis, Echinochloa crus-gali, Eleusine coracana, Hordeum vulgare, Panicum maximum, P. capillare, Pennisetum glaucum, Sorghum bicolor, S. sudanense, Zea mays
Geographic distribution
East Africa, West Africa, South America
Côte d’Ivoire, Brazil, Colombia
Biology and transmission
This virus is related to Johnson grass mosaic virus
Transmitted by aphids and through mechanical inoculation
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse to re-establish plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Virus of Plants. Description and List from the VIDE Database. CAB International, UK. 1484 pp.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecct. 800 pp.
Lenne JM, Trutmann P. (eds.) 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & Centro Internacional de Agricultura Tropical (CIAT), Colombia. 404 pp.
Olufemi WA, Mbiele Al, Nkouka N. (eds.) 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Seed Health General Publication Published by the Center or CGIAR
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Scientific name
Johnsongrass mosaic virus (JGMV)
Other scientific names
Maize dwarf mosaic virus — strain O (McDaniel and Gordon, 1985; Shukla et al., 1989),
Sugarcane mosaic virus — Australian Johnson grass virus (Shukla et al., 1987),
Maize dwarf mosaic virus — Kansas I strain (McKern et al., 1990).
Significance
Important disease of many forage grasses, sorghum and maize but of quarantine significance because currently not widely distributed.
Symptoms
Mosaic and variegation, ringspots and chlorosis, necrotic red stripe, necrotic red leaf; necrosis of new leaves, stunting; systemic chlorotic mosaic, mottling, necrosis, stunting
Mosaic, ring spots, stunting
Hosts
Brachiaria miliiformis, B. praetervisa, Cenchrus ciliaris, Panicum miliaceum, Paspalum orbiculare, Pennisetum typhoides, Sorghum x almum, S. bicolor, S. haplense, S. laxiflorum, S. macrospermum, S. miliaceum, S. stipoideum, S. sudanense, S. verticilliflorum, S. vulgare, Zea mays
Geographic distribution
USA, Australia, East Africa
Biology and transmission
Transmission by aphids, sap and mechanical inoculation
Vascular puncture inoculation of seedlings
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse and screen for disease free seedlings to re-establish plots
References and further reading
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Scientific name
Maize dwarf mosaic virus (MDMV)
Other scientific names
MDMV-A, MDMV-D, MDMV-E, MDMV-F; Sugarcane mosaic virus (SCMV), Sorghum red stripe virus (SRSV).
Significance
Widespread, seed and vector transmitted and also affects maize and sorghum.
Symptoms
Uneven chlorotic stripes in leaves, occasional reddening; foliar faint streak, mottle, foliar ring-like flecks; distortion and necrosis of young leaves; poor filling of cobs, stunting
Mosaic and stunting
Hosts
Avena sativa, Brachiaria eruciformis, B. platyphylla, Chloris gayana, Cynodon dactylon, Dactylis glomerata, Digitaria sanguinalis, Echinochloa crus-gali, Eleusine coracana, Eragrostis trichodes, Hordeum vulgare, Lolium perenne, Melinis minutiflora, Panicum maximum, P. sumatrense, P. capillare, Paspalum dilatatum, Poa pratensis, Rottboellia cochinchinensis, Secale cereale, Setaria viridis, Sorghum arundinaceum, S. bicolor, S. haplense, Zea mays
Geographic distribution
Australia, China, South Africa, USA
Biology and transmission
Transmitted by aphids; seed-borne
In Africa Zea mays is grown in mid to high altitudes.
Did not affect oats, rice, wheat, soybeans and cowpeas.
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: ELISA, TBIA.
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse and screen for disease free seedlings to re-establish plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Virus of Plants. Description and List from the VIDE Database. CAB International, UK. 1484 pp.
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecct. 800 pp.
Lenne JM, Trutmann P. (eds.) 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & Centro Internacional de Agricultura Tropical (CIAT), Colombia. 404 pp.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Olufemi WA, Mbiele AL, Nkouka N. (eds.) 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Scientific name
Maize streak virus (MSV)
Other scientific names
Bajra streak virus (Seth et al., 1972a & 1972b), Cereal African streak virus, Maize streak A virus
Significance
Causes severe streaking and yield loss in maize and other grasses.
Quarantine significance because not found in the Americas.
Symptoms
White chlorotic spots along veins of unfolding leaf; chlorotic streaking to uniform chlorosis; necrosis, local lesions, stunting; chlorotic or white streaking or lesions; systemic chlorotic streaking
Chlorotic streaking and various other foliar lesions.
Hosts
Agropyron cristatum, A. fragile, Agrostis gigantean, Andropogon gerardii, Arrhenatherum elatius, Avena sativa, Axonopus compressus, Bothriochloa barbinodis, Brachiara deflexa, B. lata, B. reptans, B.villosa (B. distichophylla), Bromus erectus, B. inermis, B. catharticus, Cenchrus ciliaris, Chloris gayana, Coix lacryma-jobi, Cymbopogon schoenanthus, Cynodon dactylon, Dactylis glomerata, Dactyloctenium gianteum, Digitaria abyssinica, Digitaria velutina, D. milanjiana, D. ternata, D. eriantha, D. horizontalis, D. sanguinalis, Echinochloa colona, E. crus-gali, E. polystachya, Eleusine coracana, E. indica, Eragrostis curvula, Fesuca ovina, F. pratensis, F. rubra, Heteropogon contortus, Holcus lanatus, Hordeum vulgare, Hyparrhenia rufa, Leersia hexandra, Lolium multiflorum, L. perenne, L. rigidum, Panicum coloratum, P. maximum, P. bergii, P. sumatrense, Paspalum dilatatum, P. notatum, P. scorbiculatum, P. almum, P. urvillei, Pennisetum clandestinum, P. purpureum, P. glaucum, Phalaris aquatica, P. arundinacea, Phleum pretense, Poa pretense, Rottboellia cochinchinensis, Setaria sphacelata, S. pumila, S. verticillata, S. megaphylla, S. homonyma, S. viridis, Sorghum arundinaceum, S. bicolor, Tripsacum dactyloides, Urochloa panicoides, U. trichopus, Zea mays
Geographic distribution
Madagascar, East Africa, Yemen, Reunion, India
Biology and transmission
This virus is transmitted by insects belonging to Cicadelllidae (Cicadulina mbila, Cicadulina triangular, Cicadulina zeae, Cicadulina storeiy) in a persistent manner; transmissible to seedlings by vascular puncture inoculation (VPI), but not through seed or by mechanical means; non-sap transmissible.
Detection/indexing method
- at CIAT: not applicable
- at ILRI: ELISA, TBIA
Treatment/control
- Chemical control of the leafhopper vector is only justified for special purposes such as germplasm or seed muliplication.
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse and screen for disease free seedlings to re-establish plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Virus of Plants. Description and List from the VIDE Database. CAB International, UK. 1484 pp.
Diekmann M, Putter CAJ. (eds.) 1995. FAO/IPGRI Technical Guidelines for the Safe Movement of Germplasm. No. 14. Small Grain Temperate Cereals. Food and Agriculture Organization of the United Nations, Rome/International Plant Genetic Resources Institute, Rome.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecct. 800 pp.
Lenne JM, Trutmann P. (eds.) 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & Centro Internacional de Agricultura Tropical (CIAT), Colombia. 404 pp.
Miles JW, Maass Bl, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Olufemi WA, Mbiele AL, Nkouka N. (eds.) 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Scientific name
Sugarcane mosaic virus (SCMV)
Other scientific names
Grass mosaic virus, Maize dwarf mosaic virus MDMV-B MDMV-A, Sorghum red stripe virus (SRSV)
Significance
Worldwide spread and yield loss in some forages.
Symptoms
Foliar mottling; general mosaic to oblong chlorotic spots; oblong necrotic spots dispersed in leaves; necrotic local lesions, then systemic mosaic, necrosis
Mosaic in different variegated patterns, depending on the age of the plant and time of inoculation.
Hosts
Brachiaria eruciformis
Brachiaria spp.
Geographic distribution
Worldwide
Australia, East Africa
Biology and transmission
Transmitted by aphids; spread through infected seeds, sap, vegetative propagules and by mechanical means
Transmissible to seedlings by vascular puncture inoculation (VPI)
Detection/indexing method
- at CIAT: Not applicable
- at ILRI: NASH, PCR method
Treatment/control
- None
Procedure followed at the centers in case of positive test
- For low infection, rogue the field and burn infected plants. For high infection produce seeds in a screenhouse and screen for disease free seedlings to re-establish plots.
References and further reading
Alan B, Crabtree K, Dallwitz M, Gibbs A, Watson L. (eds.) 1996. Virus of Plants. Description and List from the VIDE Database. CAB International, UK. 1484 pp.
Frison EA, Putter CAJ. (eds.). 1993. FAO/IBPGR Technical Guidelines for theSafe Movement of Sugarcane Germplasm. Food and Agriculture Organization of the United Nations, Rome/ International Board for Plant Genetic Resources, Rome.
Gad L, Thottappilly G. (eds.) 2003. Virus and Virus-like Diseases of Major Crops in Developing Countries. Kluwer Academic Publishers, Dordrecct. 800 pp.
Lenne JM, Trutmann P. (eds.) 1994. Diseases of Tropical Pasture Plants. CAB International, UK, Natural Resource Institute, UK & Centro Internacional de Agricultura Tropical (CIAT), Colombia. 404 pp.
Miles JW, Maass BL, do Valle CB; with the collaboration of V. Kumble. (eds.) 1996. Brachiaria: Biology, Agronomy and Improvement. Cali, Colombia: Centro Internacional de Agricultura Tropical, Tropical Forages Program and Communications Unit; Campo Grande, Brazil, Empresa Brasileira de Pesquisa Agropecuaria, Centro National de Pesquisa de Gado de Corte, 1996. 288 p. CIAT Publication; no. 259
Olufemi WA, Mbiele AL, Nkouka N. (eds.) 1988. Virus Diseases of Plants in Africa. Organization of African Unity/Scientific, Technical & Research Commission (OAU/STRC), Technical Center for Agricultural & Rural Cooperation: Lagos Nigeria. 225 p.
Sukumar C, Leath KT, Skipp RS, Pederson GA, Bray RA, Latch GCM, Jr Nutter FW. (eds.) Pasture and Forage Crop Pathology. American Society of Agronomy, Inc. Crop Science Society of America, Inc., Soil Science Society of America, Inc. 653 pp.



 stog-forage-grass
stog-forage-grass