CGKB News and events main
Regeneration guidelines for small-grained cereals
|
View regeneration guidelines in full (in PDF)
Also available in the following languages:
|
The information on this page was extracted from:
Payne T.S., Amri A., Humeid B. and Rukhkyan N. 2008. Regeneration guidelines: small-grained cereals. In: Dulloo M.E., Thormann I., Jorge M.A. and Hanson J., editors. Crop specific regeneration guidelines [CD-ROM]. CGIAR System-wide Genetic Resource Programme, Rome, Italy. 12 pp.
Before reading the regeneration details for this crop, read the general introduction that gives general guidelines to follow by clicking here.
Introduction
Small-grained cereals include bread wheat (Triticum aestivum L.), spelt (T. spelta L.), durum wheat (T. durum Desf.), emmer (T. dicoccon Schrank), triticale (x Triticosecale spp.), barley (Hordeum vulgare L.) and oats (Avena sativa L.). These cereals are self-pollinated, annual, cool-season grasses that are grown for human food, animal feed and forage.
All of these species are found as both spring-and winter-planted types. Winter types require vernalization; cold tolerance is highest in wheat (down to -25°C), lower in barley (-20°C) and lowest in oats (-15°C). Accessions may be wild species, landraces, obsolete improved varieties, advanced improved varieties, breeding materials or genetic stocks and may be maintained as populations or as pure-breeding lines. Rye (Secale cereale L.) is excluded from this guideline because it requires specialized regeneration procedures due to its cross-pollinated reproductive nature.
 Bread wheat (Triticum aestivum L.) landraces being characterized for drought tolerance, Obregon, Mexico (photo: Ana Maria Sanchez/CIMMYT) |
Choice of environment and planting season
Planting season
- In dry areas with winter season precipitation, plant at the start of the rainy season.
- Select date of planting and seeding rate according to recommended agronomic practices or local farmers’ best practices.
- Planting too early may produce excessive early-season growth and use large amounts of moisture and nutrients but if moisture, nutrients and hot temperatures are not limiting, early planting normally results in higher yields. Later planting can improve seed quality.
- Early planting, however, can allow plants to take advantage of available moisture and may avoid terminal drought stress.
- Planting too late may increase the possibility of wind erosion due to poor ground cover and the danger of cold damage for autumn-sown cereals. Later plantings will mature a few days later.
Preparation for regeneration
Maintaining population integrity
When conserving and regenerating accessions that are populations of genetically diverse individuals, it is important to maintain adequate seed numbers (at least 500 viable seeds) to capture the full range of variation and genetic integrity, preventing the effects of genetic drift.
When to regenerate
- Regenerate accessions if the germination or viability rate is less than 85% or the number of viable seeds in the active collection falls below 1100.
- Newly introduced, collected or received materials require regeneration to meet international standards of seed amount and quality.
- Accessions with an unknown multiplication origin require regeneration to enable documentation of phytosanitary origin, purity and cleanliness.
Pre-treatments
- Treat seed with fungicides and insecticides if required. Use local recommendations as a guide to types and rates of chemical application.
Field selection and preparation
- Preferably use a field in which the previous crop was a non-cereal crop, or that had been fallow.
- Fence fields to prevent grazing, locate them away from bird roosts and rodent dens and remove any noxious weeds.
- Plough and disc-cultivate soils prior to sowing to prepare a uniform seed bed.
|
Using Jiffy pots for rescue regeneration of Barley and wild relatives (photo: ICARDA)
Barley regeneration plot (photo: ICARDA) |
Artificial vernalization
Accessions with a strong vernalization requirement will require a refrigerated vernalization treatment if field conditions do not provide cool enough temperatures for long enough (<5°C for 6–8 weeks) to satisfy their vernalization requirements. Conduct the artificial vernalization treatment 8–10 weeks before the optimum field seed sowing date to allow transplanted seedlings optimal plant growth and development in the field.
- Identify genotypes that require vernalization treatment.
- Treat seeds with a fungicide.
- Place seeds on moistened blotter paper and allow germination to begin at ambient temperatures.
- Place the papers into individual Petri dishes or clear plastic bags sealed with a twist-tie.
- Refrigerate at 1–3°C with a light source (8 hours/day) and keep blotter paper moist.
- The period of cold treatment may be as short as 4 weeks in accessions with a medium vernalization response, while those with a strong vernalization requirement may need 6–8 weeks of cold treatment.
- Carefully transplant seedlings into the field on completion of the vernalization cold treatment.
Wild cereal species
Germination of wild species tends to be much more irregular than that of cultivated seed. The following measures are recommended to encourage germination of seeds of wild relatives:
- In the case of husked seeds, remove seeds from husks.
- Consider germinating in Petri dishes if few seeds are available and transplant seedlings to pots or into the field.
NOTE: Wild species often have vernalization requirements.
NOTE: Seeds in lower florets will germinate first.
NOTE: Wild species often have day-length (long day) sensitivity and so require early planting.
Method of regeneration
- Use single replicate trials for seed regeneration and plant local check varieties at standard intervals (e.g. every 20th plot). Local check varieties should be adapted to the multiplication environment with a phenology and stature (flowering, maturity and plant height) allowing relative characterization measurements with adjacent plots.
Planting layout, density and distance
 Wild red oat (Avena sterilis L.) plots separated by sunflowers to protect against wind (photo: Axel Diederichsen/Plant Gene Resources of Canada) |
- Use six-row plots with 15–30 cm between rows.
- Harvest only the centre four rows for seed.
- Determine the plot length according to the amount of seed needed to fulfil all conservation and testing (phytosanitary, germination, etc.) requirements and the anticipated grain yield. (NOTE: a grain yield of 1 t/ha is equal to 100 g/m2 ).
- For tall and lodging-susceptible materials, leave a gap of 90 cm between plots or plant alternate plots with non-cereal crop species to avoid entangled culms and subsequent grain mixtures between accessions during harvest.
- Landraces and wild relatives may be especially prone to lodging under cultivated conditions, so grow them in a place protected from the wind (see photo) or in a tunnel, and stake plants as necessary.
Sowing rate
- Use a seed rate equivalent to 125–250 viable seeds/m2 (approximately 5–10 g/m2 or 5–10 g seed per 3-m row).
Labelling
- Place the seeds to be planted in packets labelled with genebank code number and plot identification number.
- Prepare a planting map prior to sowing, and immediately after sowing note on the map any planting errors committed, and record the sowing date. Mark first and final nursery plots with stakes at the time of sowing.
- Prepare a field book that lists the nursery name, plot number with corresponding germplasm name and accession number, and accession seed source. Record characterization and evaluation data in this field book.
- Label or tag each row with the nursery name and plot number. Use weather-proof labels and ink.
- Erect boards that have a brief description of the nursery and its contents to raise public awareness of your activities among colleagues and administrators as well as among local farmers, visitors and the media.
- Bar-coding and the use of palm-tops (hand-held field data recording equipment) can reduce errors and facilitate computerized management and control.
Crop management
When in doubt, follow locally recommended best crop husbandry practices, including date and rate of planting, amounts and timing of fertilizer and supplementary irrigation applications, weed, disease and pest control, and time of harvest and post-harvest seed storage. The goal is to maintain accession integrity while producing ample quantities of highly viable, sound grain.
Weed management
- Prepare a weed-free seed bed immediately prior to sowing.
- Avoid unnecessary herbicide treatment that could compromise the genetic integrity of accessions. Diverse genebank materials may react very differently to herbicides than cultivated materials. Control weeds throughout the growing cycle.
- Take particular care to exclude quarantined weed species from the regeneration nurseries and fields.
Irrigation
- In areas with less than 500mm annual available rainfall, supplementary irrigation may be required.
- If irrigation facilities are available, apply irrigation at least twice to establish a full soil moisture profile: a) immediately after sowing; b) immediately prior to flowering (e.g. during booting).
- In areas with high annual rainfall and also when irrigation is used, avoid sowing plots in areas of the field prone to waterlogging.
Fertilization
- Apply a fertilizer balanced for nitrogen, phosphorus and potassium, based on location conditions, practices, soil test results and fertilizer availability.
- Landraces, tall materials and wild relatives are not adapted to high nitrogen levels used in modern agriculture. For these materials, use low levels of nitrogen fertilizer. Apply 60% of the recommended local farmer rates to avoid excess plant growth, which can lead to lodging and heavy mildew infection.
 Wild oat panicle in a glassine bag to prevent loss of seed (photo: Axel Diederichsen/Plant Gene Resources of Canada)
Clean the plot combine after use! (photo: ICARDA) |
Pest and disease control
- Field crop rotation is often the best means of managing persistent pests and diseases.
- Use seed- and foliar-applied fungicides, herbicide and bird and rodent protective devices, as required, and follow integrated crop protection and management recommendations.
Roguing off-types
- Take care when roguing off-type plants from within an accession by only discarding plants that are known to be contaminants or volunteers. Monitor for off-type plants several times throughout the growing season.
For accessions that are prone to shattering
- To prevent seed loss due to shedding and shattering, bag ears or panicles during ripening using perforated plastic or glassine bags and fix the bags to stakes with pegs for support (see photo).
Harvesting
- Prior to harvest, prepare cloth or paper harvest bags labelled with the genebank code number and plot identification number.
- Harvest when spikes are mature, i.e. when 90% of spikes in the plot are yellow and the grains are hard when pressed between finger nails.
- Harvest only the four centre rows (row ends trimmed) of each six-row plot.
- Check that the information on the bag label and plot tag are the same. Place the seed harvested from a plot and the plot tag into the labelled harvest bag.
- To reduce the risk of bird damage and shattering, or pre-harvest sprouting in high rainfall conditions, cut and bundle each accession no later than one week after its maturity. Label each bundle with plot or harvest tags including the genebank code number and plot identification number. Dry bundles in a well ventilated, covered location.
- Thresh the seed using a stationary thresher or a plot combine.
- Clean the thresher or plot combine meticulously after harvesting each plot.
- Dry the grain using ambient, non-heated air to a uniform moisture content of 12% before grain weights are recorded and the grain is stored for further processing.
- Take particular care when threshing hull-less barley and naked oats as the germ is often more susceptible to mechanical removal or damage than is the case with hulled cereals or wheat.
- Spontaneous seed shattering and uneven maturity in wild relatives may require repeated harvesting of individual accessions by hand in order to harvest individual plants at the optimum maturity and reduce seed loss. Harvest spikes of these materials early in the morning, every second day.
Post-harvest management
Seed cleaning
- Clean threshed grain of chaff, straw, diseased seed, broken seed, weed seed and soil using air-blown seed cleaners.
- Meticulously clean the seed cleaner after cleaning each accession.
- Fumigate seed to prevent insect damage prior to cold storage.
Seed drying
- Primary drying for short-term storage: Keep harvested seed in cloth or paper bags in a dry location protected from rain and rodents. Dry seed in a well-ventilated room at not more than 35°C to a seed moisture content of 12–15%.
- Secondary drying for long-term storage: Place cloth or paper bags in a cool dry room at 10–25°C and 10–15% relative humidity for 6–8 weeks until seed reaches an equilibrium moisture content of 5–8%. If a drying room is not available, dry seeds to a moisture c ontent of 5–8% with silica gel or another appropriate desiccant.
Seed packaging
-
Pack seed in containers or packets that are impervious to air and moisture (preferably in laminated aluminum foil packets for long-term base collections or screw-topped plastic or glass bottles for medium-term, active collections).
- Long-term collections should contain about 1250–2500 seeds, or approximately 50–100 g of seed, per accession.
- Medium-term, active collections should contain about 5000–7500 viable seeds (200–300 g), or more in the case of genetically heterogeneous accessions.
- Safety duplicate sample(s) should contain about 500 seeds, or 10–20 g of seed.
- Express excess air from the packet or container and then seal it hermetically.
- During seed processing, check seed characteristics against the passport and characterization data to ensure the correct accession identity by taxon, seed texture and colour.
- Do not use fungicides or insecticides for seed storage as these chemicals often reduce seed viability in longer-term storage conditions.
- Pack a standard number of packets or containers into boxes and code each box for its contents and storage location within the storage facility.
- Bar-coding of individual packets/containers facilitates genebank management and limits human error.
Germplasm seed health inspection
- Before incorporating accessions into your collection, check for the presence of quarantined seed-borne diseases. All accessions intended for distribution must be healthy to pass phytosanitary regulations and limit the spread of disease.
Common seed-borne pests and diseases
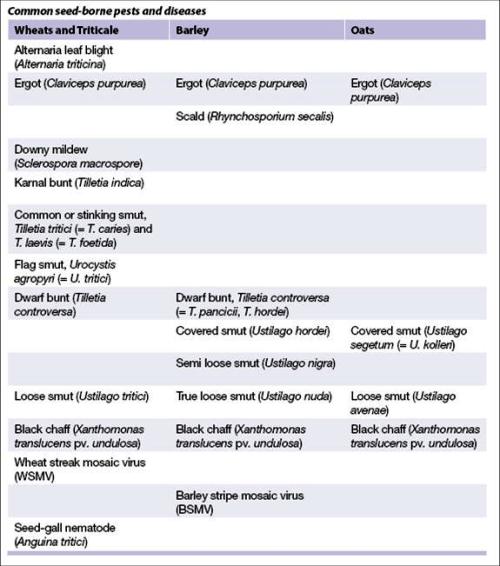
Storage conditions
- Store hermetically sealed accession packets or containers at as low a temperature as possible: 0–2°C for medium-term, active storage; -18 to -20°C for long-term storage.
Safety duplication
- The accessions in your collection are vulnerable to a wide range of threats, such as lack of adequate funding, equipment failures, regeneration errors, loss of skilled expertise, changes in institutional priorities, civil strife and natural catastrophes.
- Unique varieties are lost whenever disaster strikes, therefore securing duplicates of your collections in locations outside your institute campus provides an insurance policy for the collection and the world’s food supply.
- All genebanks are also encouraged to use the Svalbard Global Seed Vault as a ‘black box’ safety duplication repository. Seed deposit guidelines can be obtained from: This email address is being protected from spambots. You need JavaScript enabled to view it.
Monitoring accession identity
As materials are regenerated, conduct true-to-type verification using passport descriptors, comparative reference seed collections, minimum characterization traits, herbarium sheets and photo-documentation.
- The accession should be discarded if its characteristics do not match the original accession.
- Store phenotypically heterogeneous accessions (often the case with original landrace or wild relative populations) as obtained.
- During the initial multiplication, take separate head-rowed subsamples (individual spikes from distinct plants) and regenerate them separately.
- Identify subsampled pure lines as derivatives from the initial heterogeneous accession, linked through the original progenitor accession identification number.
- If doubts arise as to the number of subsamples to be taken, err on the high side to ensure maintenance of the greatest amount of potentially useful diversity from the original population.
Documentation of information during regeneration
Collect the following information during regeneration, based on multi-crop passport descriptors (FAO/IPGRI 2001):
- Donor’s details (number, name, institution, country).
- Germplasm acquisition agreement (GAA) status.
- Standard materials transfer agreement (SMTA) or MTA status.
- Genus, species and subspecific taxonomy.
- Accession identification number(s).
- Name(s).
- Accession ancestral pedigree, cross and selection historical data.
- Biological status: wild, traditional cultivar or landrace, breeding or research material, improved cultivar, other, or unknown.
- Country of origin.
- Collection mission data.
- Collector or breeder’s name and affiliations.
- Collecting or breeding number.
- Collection site latitude, longitude and elevation.
- Collection site description.
- Date and location of regenerations.
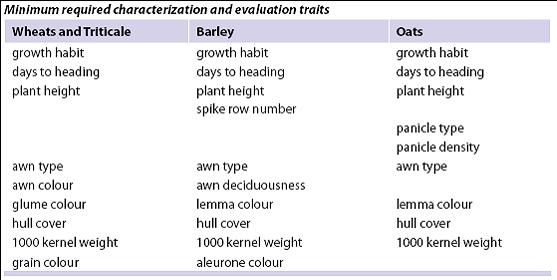
Standard methods and scale for the reporting descriptor traits can be found under the respective crop ‘List of Descriptors’ at: http://www.ars-grin.gov/cgi-bin/npgs/html/croplist.pl.
References and further reading
Breese EL. 1989. Regeneration and multiplication of germplasm resources in seed genebanks: The scientific background. IBPGR, Rome, Italy.
Cook RJ, Veseth RJ. 1991. Wheat Health Management. APS Press, The American Phytopathological Society, St. Paul, MN, USA.
Engels JMM, Visser L, editors. 2003. A guide to effective management of germplasm collections. IPGRI Handbooks for Genebanks No. 6. IPGRI, Rome, Italy. Available in English (1.4 MB) and Spanish (1.5 MB).
FAO/IPGRI. 1994. Genebank standards. Food and Agriculture Organization of the United Nations, Rome and International Plant Genetic Resources Institute, Rome. Available in English, Spanish, French and Arabic .
FAO/IPGRI. 2001. Multi-Crop Passport Descriptors. FAO and IPGRI, Rome, Italy. Available in English, French and Spanish.
IBPGR. 1985. Oat descriptors. IBPGR, Rome, Italy.
IBPGR. 1985. Descriptors for wheat (Triticum spp.), revised. IBPGR, Rome, Italy. Available here
IPGRI. 1994. Descriptors for barley (Hordeum vulgare L.). IPGRI, Rome, Italy. Available here.
ISTA. 2008. International Rules for Seed Testing. ISTA Secretariat, Switzerland.
Lehmann ChO, Mansfeld R. 1957. Zur Technik der Sortimentserhaltung [On the technique for collection-maintenance]. Kulturpflanze 5:108–138.
Mathre DE, editor. 1997. Compendium of Barley Diseases, 2nd edition. APS Press, The American Phytopathological Society, St. Paul, MN, USA.
Mezzalama ML, Gilchrist L, McNab A. 2001. Seed health: rules and regulations for the safe movement of germplasm. CIMMY T, Mexico City, Mexico.
Wiese MV. 1987. Compendium of Wheat Diseases, 2nd edition. APS Press, The American Phytopathological Society, St. Paul, MN, USA.
Acknowledgements
The author wishes to acknowledge the inputs of Harold Bockelman, USDA Small Grains Collection, USA; Monica Mezzalama, International Maize and Wheat Improvement Center (CIMMY T) Seed Health Laboratory, Mexico; and Imke Thormann, Bioversity International, Italy. These guidelines have been peer reviewed by Axel Diederichsen, Plant Gene Resources of Canada; Christoph U. Germeier, Julius Kuehn Institute, Federal Research Centre for Cultivated Plants, Germany; Igor Loskutov, N.I. Vavilov Research Institute of Plant Industry (VIR), Russia; and Jan Valkoun, International Center for Agricultural Research in the Dry Areas (ICARDA), Syria (retired).



 main
main




