Fungi - sorghum
Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
|
Contents: |
|
Ergot (Claviceps sorghi) of sorghum: Honeydew stage |
|
Ergot (Claviceps sorghi) of sorghum: Sclerotial stage |
|
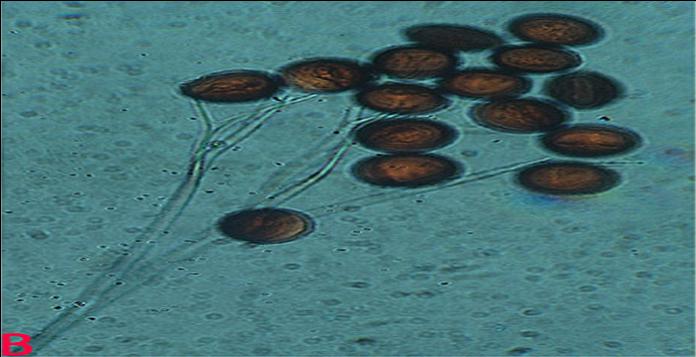
Ergot (Claviceps sorghi) of sorghum: Sclerotia |
Scientific name
Claviceps sorghi Kulkarni Seshadri & Hegde.
Other scientific names
Claviceps americanum; Claviceps africanum
Importance
High
Significance
Yield losses of 10-80% occurred hybrid seed production in India and regular annual losses of 12-25% recorded in Zimbabwe. It has been estimated that ergot costs the Australian seed industry A$4 million annually and the annual production cost increased by $5 million due to ergot in USA (Bandyopadhyay et al. 1998).
Symptoms
Individual ovaries of florets are replaced by a soft, white, subglobose-shaped growth of mycelium (sphacelium) from which sticky, liquid droplets of spore-bearing honeydew (thin to viscous, orange-brown or superficially white) will exude. Under favorable conditions long (1-2 cm) straight or curved, cream to light brown, hard sclerotia developed (Williams et al. 1978).
Hosts
Sorghum bicolor (common sorghum) and Sorghum halepense (aleppo grass).
Geographic distribution
Ergot has been reported from many sorghum growing countries in Asia, Africa, USA and in Latin America (Frederickson et al. 1989).
Biology and transmission
The pathogen has sclerotial and sphacelial stages in its life cycle. The sclerotia produces ascospores which cause primary infection in the florets. The infected florets starts oozing of honeydew which contains mostly microconidia. The microconidia are round to oval and reproduces by either budding or by producing germtubes bearing secondary conidia. The secondary and tertiary conidia are the virulent propagules for causing the infection. Under conditions of high relative humidity, macroconidia at the honeydew surface germinate iteratively to produce aerially-supported secondary conidia, rendering the honeydew white in appearance (Frederickson et al. 1989). Sclerotia mixed with the seed cause the primary infection. Secondary conidia are wind-borne spores for causing epiphytotics. Sometimes insects will carry the conidia from plant to plant, and rain splashes also plays an important role in spreading the disease.
Detection/indexing methods
- At ICRISAT - Pre export field inspection and dry seed examination
Treatment/control
- Sclerotia from the seed lots are removed manually, and the seed samples are treated with captan (3g kg-1 seed) to inactivate any conidia attached to the seed surface (Odvody et al. 2000).
Procedures followed at the centers in case of positive test
- At ICRISAT all infected samples are incinerated if they are infected in field. Sclerotia are separated manually if they come as seed-mix, and the seeds are treated with captan (3g kg seed-1) to inactivate any conidia attached to the seed surface.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Bandyopadhyay R, Frederickson DE, McLaren NW, Odvody GN, Ryley MJ. 1998. Ergot: a new disease threat to sorghum in the Americas and Australia. Plant Disease 82: 356-367.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Frederickson DE, Mantle PG, Milliano WAJde. 1989. Secondary conidiation of Sphacelia sorghi on sorghum, a novel factor in the epidemiology of ergot disease. Mycological Research 93: 497-502
Odvody GN, Frederickson DE, Isakeit T, Montes N, Dahlberg JA, Peterson GL 2000. Quarantine issues arising from contamination of seed with ergot: an update. In: Global 2000, sorghum and pearl millet diseases III, 23-30 September 2000, Guanajuato, Mexico.
Williams RJ, Frederiksen RA, Girard JC. 1978. Sorghum and pearl millet disease identification handbook. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics. 88 pp.
|
Milo disease (Periconia circinata) of sorghum: Symptoms on |
|
Milo disease (Periconia circinata) of sorghum: Conidiophores |
Scientific name
Periconia circinata (L). Mangin Sacc.
Other scientific names
Aspergillus circinatus.
Importance
High.
Significance
Losses of up to 50-60% from Milo disease on susceptible varieties grown on infested soil.
Symptoms
Lesions occur on fine lateral roots, secondary roots and prop roots of sorghum. In an advanced stage periconia rot is characterized by red discoloration of the stele accompanied by flecking with brown host cells from which conidiophores of P. circinata arises. Conidia and conidiophores can be seen on the stele and root epidermis. Sometimes the seedlings are stunted in susceptible genotypes and the leaves tend to be curly. The crowns of diseased plants, when split, show a dark red discoloration. The infected plants may develop subnormal grains (Mayers 1976).
Hosts
Sorghum bicolor (common sorghum), Sorghum almum (Argentinegrass) and Sorghum halepense (aleppo grass).
Geographic distribution
Wide spread in South Africa. Reported in Australia, France and USA (Mayers 1976).
Biology and transmission
Colonies are small and compact, gray, brown, olivacious brown or black, hairy. Mycelium mostly immersed but sometimes partly superficial. Stroma frequently present, mid to dark brown, pseudoparenchymatous. Conidiophores are mostly macronematous with stipe and spherical head, looking like rounded pins, pale to dark brown, often appearing black and shining by reflected light. Conidia catenate, chains, arising at one or more points of the curved surface of the conidiogenous cell which are monoblastic and poly blastic. Conidia are simple, usually spherical or sub-spherical, occasionally ellipsoidal, oblong or broadly cylindrical, pale to dark brown, verruculose with no septa. P. circinata is both seed borne (Mayers 1976) and soil borne (Odvody et al. 1977).
Detection/indexing methods
- At ICRISAT - Pre export field inspection.
Treatment/control
- No seed treatment is suggested since it is not available.
Procedures followed at the centers in case of positive test
- So far not detected.
References and further reading
Mayers PE. 1976. The first recordings of Milo Disease and Periconia circinata on sorghum in Australia. Australian Plant Pathology Society Newsletter 5: 59-60.
Odvody GN, Dunkle LD, Edmunds LK. 1977. Characterization of the Periconia circinata population in a milo disease nursery. Phytopathology 67: 1485–1489.
|
Downy mildew (Perenosclerospora sorghi) of sorghum: |
|
Downy mildew (Perenosclerospora sorghi) of sorghum: |
Scientific name
Peronosclerospora sorghi Weston and Uppal (Shaw).
Other scientific names
Sclerospora andropogonis-sorghi, Sclerospora graminicola var. andropogonis-sorghi, Sclerospora sorghi, Sorosporium andropogonis-sorghi
Importance to CGIAR centers
Medium.
Significance
Sorghum downy mildew is economically important and widespread in many tropical and subtropical regions of the world. The disease is highly destructive due to its systemic nature of the infection resulting in death of the plants or lack of grain formation in the panicles.
Symptoms
Both systemic and local infections occur. Symptoms appear as chlorotic foliage. The first infected leaf shows chlorosis on the lower part of the lamina, which further grows to cover large part of the leaves. The other leaves on a plant that get infected subsequently show more chlorosis. Under cool and humid weather conditions, the abaxial surface of the chlorotic leaves produce abundant conidia that appear as white downy growth. As the plant grows the infected leaves start shedding. The local lesions on foliage are the result of the infection by conidia. These appear as stippled, necrotic lesions on leaf blades.
Hosts
Sorghum bicolor (common sorghum), S. caffrorum (African millet), S. sudanense (sudan grass), S. arundinaceum (common wild sorghum), S. halepense (johnsongrass), Zea diploperennis (Diploperennial teosinte), Zea mays (maize), Zea mexicana (teosinte), Andropogon sorghi (jowar) and Panicum trypheron (aleppo grass).
Geographic distribution
Worldwide.
Biology and transmission
The pathogen has sexual spores, oospores which are smooth, globose, spherical and dark brown. The asexual spores, conidia are hyaline, round to oval with papillae. The conidiophores are dichotomously branched with pointed sterigmata bearing conidia (Pande et al. 1997). Initial infections can occur from oospores in the soil and also from conidial showers from infected leaves. In certain regions, the perennial wild grasses, Johnson grass (Sorghum halepense and S. arundinaceum), are reservoirs of infection and provide primary sources of inoculum. Secondary spread of the disease occur through wind-borne conidia.
Detection/indexing methods
- Pre export field inspection and seed washing test. The Quarantine procedures for sorghum downy mildew has been developed (Chakrabarty et al. 1998).
Treatment/control
- Seed treatment with metalaxyle (2 g a.i. kg-1 seed).
Procedures followed at the centers in case of positive test
- Incineration of infected samples in the field, and rejection of seed samples with positive test under seed washing test.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seedborne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Chakrabarty SK, Prasada Rao RDJ, Varaprasad K, Singh SD, Girish GA. 1998. The Quarantine procedures for sorghum downy mildew - A Note on the past experience. Indian Journal of Plant Protection 26: 167-169.
Pande S, Bock CH, Bandyopadhyay R, Narayana YD, Reddy BVS, Lenne JM, Jeger MJ. 1997. Downy mildew of sorghum. Information Bulletin No. 51. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics. pp 32.
|
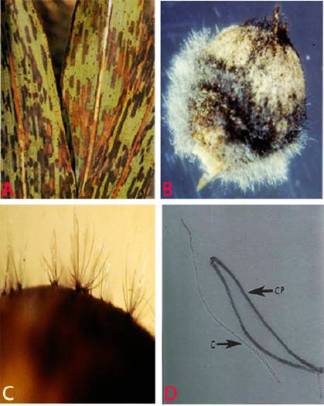
Anthracnose (Colletotrichum graminicola) of sorghum. A. lesions on leaf; B. infected seeds; C. acervuli on seed and D. conidia. (photos: ICRISAT) |
Scientific name
Colletotrichum graminicola Ces.
Other scientific names
Colletotrichum cereale, Dicladium graminicola, Steirochaete graminicola, Vermicularia melicae
Importance
Low.
Significance
Anthracnose causes severe foliage damage, resulting in 46% yield loss in West Africa and it exceeded 50% grain loss in USA. In India the grain yield loss was reported upto 16% (Thomas et al. 1996).
Symptoms
Anthracnose on sorghum affects the leaves, stems, peduncles, panicles and seeds Typical anthracnose symptoms are circular-elliptical dark spots, sometimes with a red pigmentation, which vary in size from 2 mm to more than 2 cm. The centre of mature lesions is straw-colored and contains numerous acervuli containing black seta. Under humid conditions, grey/cream/salmon-colored spore masses are produced. In many instances leaves can be entirely blighted, and when it attacked the stem it is known as 'stalk rot' (Thakur et al. 2007).
Hosts
Sorghum bicolor (common sorghum), Poaceae (cereals) and Zizania aquatica (annual wild rice).
Geographic distribution
Anthracnose has been observed in most regions of the world where sorghum is grown successfully. It is most prevalent in warm humid regions, notably in many countries of Africa, the southern states of the USA, the northeastern countries of South America, India and Indonesia (Frederiksen 1982 ).
Biology and transmission
Acervuli are irregular in shape and consist of setae. Setae are brown with dark swollen base and pale rounded tip. The acervuli consists of a gelatinous salmon orange colored conidial mass. Individual conidia are hyaline, single-cell, falcate, with acute apices. It is soil borne, seed borne and wind borne.
Detection/indexing methods
- At ICRISAT - Pre export field inspection and Blotter test (Girish et al. 2001).
Treatment/control
- Seed treatment with Benomyl + Thiram at the rate 2g kg–1 seed (Ahmed and Ravinder Reddy 1993).
Procedures followed at the centers in case of positive test
- At ICRISAT - Incineration of infected samples from the field, and rejection of seed samples with positive test under blotter method.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Frederiksen RA. 1982. Disease problems in Sorghum. Pages 263-271 in: Sorghum in the Eighties. Vol 1. L. R. House, L. K. Mughogho and J. M. Peacock, eds. ICRISAT, Patancheru, Andhra Pradesh, India.
Girish AG, Singh SD, Chakrabarty SK, Prasada Rao RDVJ, Surender A, Varaprasad KS, Bramel PJ. 2001. Seed microflora of five ICRISAT mandate crops. Seed Science and Technology Journal : 29: 429-443.
Thakur RP, Reddy BVS, Mathur K. 2007. Screening techniques for Sorghum Diseases. Information Bulletin N. 76. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 92pp.
Thomas MD, Sissoko I, Sacko M. 1996. Development of leaf anthracnose and its effect on yield and grain weight of sorghum in west Africa. Plant Disease, 80: 151-153.
Sorghum covered kernel smut or Sorghum covered smut
|
Covered smut (Sporisorium sorghi) of sorghum: A. smuted |
Scientific name
Sporisorium sorghi Link in Willd.
Other scientific names
Cintractia sorghi-vulgaris, Sphacelotheca sorghi, Tilletia sorghi-vulgaris, Ustilago condensate, Ustilago sorghi, Ustilago tulasnei
Importance
Medium.
Significance
It is considered as a major economic importance where the seeds are not treated.
Symptoms
Normally in an infected panicle individual ovules are replaced by conical to oval smut sori (teliospores or chlamydospores) that are covered by persistent peridia that are larger than normal grain. Initially each sorus is covered with a light pink or silver-white membrane, which later on ruptures to reveal the brownish-black smut spores. The infected kernels break open, and the microscopic spores adhere to the surface of healthy seeds where they over winter (Thakur et al. 2007).
Hosts
Sorghum caffrorum, Sorghum dochna, Sorghum sudanense (Sudan grass), Sorghum bicolor (common sorghum).
Geographic distribution
Sorghum growing areas mainly of Africa and Asia.
Biology and transmission
Smut sori are generally smooth; oval, conical or cylindrical; and vary in size from those small enough to be concealed by the glumes to those over one cm long. They may be white, gray, or brown. Only seeedborne spores cause infection. When a smut-infested kernel is planted, the teliospores (mostly 4 to 7 microns in diameter) germinate along with the seed forming a 4-celled promycelium (epibasidium) bearing lateral sporidia . The sporidia germinate and infect the developing sorghum seedling. (Sometimes the teliospores germinate directly by producing germ tubes). Once inside the seedling, the fungus grows systemically, apparently without damaging the plant until heading. At that time, the teliospores replace kernels and are surrounded by a membrane. At maturity, the membrane ruptures releasing the teliospores to contaminate seed or soil. Soil borne teliospores are not considered important in infecting seedlings. The only sources of inoculum for covered smut of sorghum are seeds infested with teliospores of S. sorghi. (Frederiksen 2000).
Detection/indexing methods
- At ICRISAT - Pre export Field inspection, dry seed examination and seed washing test.
Treatment/control
- Rejection of the seed samples.
Procedures followed at the centers in case of positive test
- Incineration of infected samples in the field, and rejection of seed samples with positive test under seed washing test.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Frederiksen RA. 2000. Covered kernel smut. Page 21 in Compandium of Sorghum Diseases, second edition (Frederiksen RA and Odvody GN, eds). American Phytopathological Society, St. Paul, Minnesota 55121-2097, USA:APS press.
Thakur RP, Reddy BVS, Mathur. K. 2007. Screening techniques for Sorghum Diseases. Information Bulletin N. 76. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 92pp.
Gray leaf spot or Cercospora leaf spot
|
Gray leaf spot (Cercospora sorghi) of sorghum: A. leaf spot; |
Scientific name
Cercospora sorghi Ellis & Everh.
Other scientific names
None.
Importance
Medium.
Significance
Economic impact is difficult to assess because the disease appears late in the crop, as it nears maturity and other foliar diseases are commonly present as well (Odvody 1986). Yield losses due to gray leaf spot are upto 67% in Africa (Marley et al. 2001), and epidemics have occurred sporadically and in some cases have been wide spread.
Symptoms
The lesions occur mostly on leaf blades and sheaths, and are mostly isolated, but can form continuously to give long stripes. The lesions are dark purple to red with a tan or brown centre. Leaf spots which enlarge to become rectangular lesions which can be 5-15 mm long by 2-5 mm wide (Odvody 1986).
Hosts
Sorghum almum (Argentinegrass), Sorghum dochna, Sorghum halepense (aleppo grass), Sorghum sudanense (Sudan grass), Sorghum bicolor (common sorghum) and Zea mays (maize).
Geographic distribution
Cercospora sorghi occur in most areas where sorghum is grown in many countries in the world.
Biology and transmission
Conidiophores are dark brown, narrower towards tip, irregular in width, each conidiophore bearing 1-6 conidia. Conidia are multi-septate, hyaline, vesicular, straight or slightly curved with truncate base. Seed borne and wind borne in spreading the disease.
Detection/indexing methods
- At ICRISAT - Pre export Field inspection and blotter test (Girish et al. 2001).
Treatment/control
- Seed treatment with Thiram @ 2.5 g kg-1 seed (Neergaard 1979).
Procedures followed at the centers in case of positive test
- At ICRISAT - Incineration of infected samples in the field, and rejection of seed samples under positive test under blotter method.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Girish AG, Singh SD, Chakrabarty SK, Prasada Rao RDVJ, Surender A, Varaprasad KS, Bramel PJ. 2001. Seed microflora of five ICRISAT mandate crops. Seed Science and Technology Journal : 29: 429-443
Marley PS, Elemo KA, Aba DA, Onu I, Akintayo I. 2001. Reactions of sorghum genotypes to anthracnose and gray leaf spot under Sudan and Sahel savannah field conditions of Nigeria. J. Sustain. Agric. 18:105-116.
Neergaard P. 1979. Seed Pathology. Vol 1. Rev. edn. London, UK: Macmillan Press, 839pp.
Odvody GN. 1986. Gray leaf spot. Pages 11-12 in: Compendium of Sorghum Diseases. Frederiksen RA ed. American Phytopathological Society, St. Paul, Minnesota 55121-2097, USA:APS press.
|
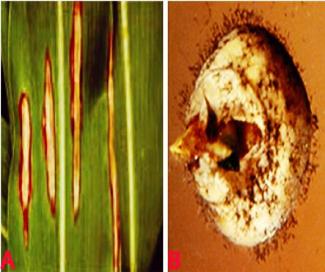
Leaf blight (Exserohilum turcicum) of sorghum. A. typical leasions on leaf; B. fungal growth on seed; C. conidiophores with conidia and D. conidia (photos: ICRISAT) |
Scientific name
Exserohilum turcicum Pass.
Other scientific names
Bipolaris turcica, Drechslera turcica, Helminthosporium inconspicuum, Helminthosporium turcicum, Keissleriella turcica, Luttrellia turcica, Setomelanomma turcica, Trichometasphaeria turcica
Importance
Medium.
Significance
Grain yield losses of up to 50% occur (Bergquist 2000).
Symptoms
Symptoms are visible from seedling stage to crop maturity stage. Small, reddish or tan spots develop on seedlings, spots later enlarge and coalesce resulting in the wilting of the young leaves. On adult plants long, elliptical, reddish purple or yellowish lesions develop, first on lower leaves and later progresses to upper leaves and stem as well. In humid weather numerous grayish black spores produced in the lesions in concentric zones (Thakur et al. 2007).
Hosts
Sorghum bicolor (common sorghum) and Zea mays (maize).
Geographic distribution
Wide spread in Asia, Africa and Americas.
Biology and transmission
The mycelium is hairy and dark gray to brown. Conidiophores are 135-335 m long and 8-10m wide. They are flexeous, simple, erect, with a swollen base and geniculated apex, and bears typically straight or spindle shaped conidia. Conidia are olivaceous brown, straightly curved and tapers towards both ends. Conidia are 3-11 distoseptate. Conidia have truncate and protuberant hilum in their basal cell. It is soil borne, wind borne and seed borne.
Detection/indexing methods
- At ICRISAT - Pre export Field inspection and blotter test (Girish et al. 2001).
Treatment/control
- Seed treatment with Benomyl + Thiram (1:1) at 2g kg-1 seed.
Procedures in case of positive test
- At ICRISAT - Incineration of infected samples in the field, and rejection of seed samples under positive test under blotter method.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeon pea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Bergquist RR. 2000. Leaf Blight, pages 9-10 in: Compendium of Sorghum Diseases, second edition (Frederiksen RA and Odvody GN, eds). American Phytopathological Society, St. Paul, Minnesota 55121-2097, USA:APS press.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Girish AG, Singh SD, Chakrabarty SK, Prasada Rao RDVJ, Surender A, Varaprasad KS, Bramel PJ. 2001. Seed microflora of five ICRISAT mandate crops. Seed Science and Technology Journal : 29: 429-443
Thakur RP, Reddy BVS, Mathur K. 2007. Screening techniques for Sorghum Diseases. Information Bulletin N. 76. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 92pp.
Ascochyta leaf spot or Rough leaf spot
|
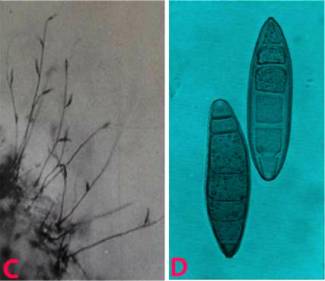
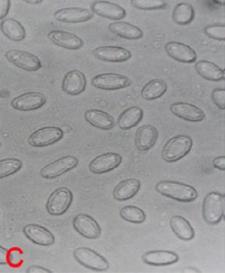
Rough leaf spot (Aschochyta sorghi) of sorghum: A. leaf spot symptoms ; B. picnidia on seed and C. picnidiospores. (photos: ICRISAT) |
Scientific name
Ascochyta sorghi Sacc.
Other scientific names
Didymella exitialis, Mycosphaerella ceres, Mycosphaerella exitialis, Sphaerella ceres,
Sphaerella exitialis
Importance
Medium.
Significance
Rough leaf spot is of minor significance in finger millet.
Symptoms
The disease appears as small, circular to oblong, light colored to reddish lesions with well-defined margins near the ends of the leaves. Pycnidia develop as small, hard, black specks in the infected areas. The lesions coalesce as they mature, and may resemble those of leaf blight, but the well-defined margins, or halos around the lesions can distinguish rough leaf spot.
Hosts
Sorghum halepense (aleppo grass), Sorghum sudanense (Sudan grass) and Sorghum bicolor (common sorghum).
Geographic distribution
Ascochyta sorghi is worldwide in distribution, especially in Europe, Asia and Africa.
Biology and transmission
Pycnidia are densely gregarious, globose-depressed, papillate, and large. Pycnidia contains numerous conidia.. Conidia are hyaline, oblong-ellipsoid, 2-celled, slightly constricted, minutely pluriguttulate and 20 8 µm (Tarr 1962). These conidia are wind borne to spread the disease. It is also reported as seedborne.
Detection/indexing methods
- At ICRISAT - Pre-export field inspection and blotter test are used.
Treatment/control
- Not known.
Procedures followed at the centers in case of positive test
- At ICRISAT - Incineration of the infected plants/seed samples.
References and further reading
Tarr SAJ. 1962. Diseases of sorghum, sudan grass and broom corn. Commonwealth Mycological Institute, Kew, England.
Comments
- No comments found











Leave your comments
Post comment as a guest